Are the knights of nanotechnology closing in on the Grail at last? A team of researchers at Stanford University claim that they are, and have chronicled their deeds in the journal Nature Nanotechnology. The valiant engineers report that they have designed a pure lithium anode.
“Of all the materials that one might use in an anode, lithium has the greatest potential. Some call it the Holy Grail,” said Yi Cui, the leader of the research team. “It is very lightweight and it has the highest energy density. You get more power per volume and weight, leading to lighter, smaller batteries with more power.”
“Lithium has major challenges that have made its use in anodes difficult. Many engineers had given up the search, but we found a way to protect the lithium from the problems that have plagued it for so long,” said Guangyuan Zheng, first author of the paper.
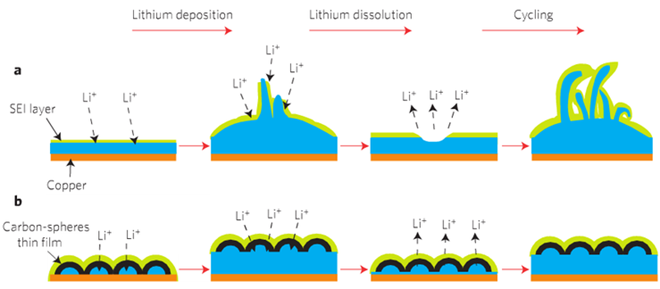
The use of lithium as an anode material has been limited by the fact that lithium ions expand as they gather on the anode during charging. All anode materials, including graphite and silicon, expand somewhat during charging, but not like lithium. Its expansion is also uneven, causing pits and cracks to form in the outer surface. The resulting fissures allow the lithium ions to escape, forming hair-like growths called dendrites, which can short-circuit the battery and shorten its life (Figure a).
Another engineering challenge is that a lithium anode is highly chemically reactive with the electrolyte. It uses up the electrolyte and reduces battery life. Yet another problem is that the anode and electrolyte produce heat when they come into contact. Lithium batteries can overheat to the point of fire, or even explosion.
To solve these problems, the Stanford researchers built a protective layer of interconnected nanospheres on top of their lithium anode. The nanosphere layer resembles a honeycomb: it creates a flexible, uniform and non-reactive film that protects the unstable lithium from the drawbacks that have made it such a challenge. The carbon nanosphere wall is just 20 nanometers thick (Figure b).
SEE ALSO: Researchers use beach sand to develop nanoscale silicon dioxide anode
“The ideal protective layer for a lithium metal anode needs to be chemically stable to protect against the chemical reactions with the electrolyte, and mechanically strong to withstand the expansion of the lithium during charge,” Cui said.
The nanospheres improve coulombic efficiency – a ratio of the amount of lithium that can be extracted from the anode when the battery is in use compared to the amount put in during charging. To be commercially viable, a battery must have a coulombic efficiency of 99.9 percent or more. Previous anodes of unprotected lithium metal achieved approximately 96 percent efficiency, which dropped to less than 50 percent in just 100 cycles. The Stanford team’s new lithium metal anode achieves 99 percent efficiency even after 150 cycles.
“The difference between 99 percent and 96 percent, in battery terms, is huge. So, while we’re not quite to that 99.9 percent threshold, where we need to be, we’re close, and this is a significant improvement over any previous design,” Cui said. “With some additional engineering and new electrolytes, we believe we can realize a practical and stable lithium metal anode that could power the next generation of rechargeable batteries.”
Source: EurekAlert! via Green Car Congress
Image: Zheng et al/Nature Nanotechnology