Their novel approach offers an advantage in terms of safety and also results in a broad (5 V wide) electrochemical window.
Researchers from Oak Ridge National Laboratory’s Center for Nanophase Materials Sciences have uncovered a new process for producing a solid state electrolyte for lithium-ion batteries. The new electrolyte has three orders of magnitude greater ionic conductivity than the same material when conventionally synthesized. Their novel approach offers an advantage in terms of safety and also results in a broad (5 V wide) electrochemical window. Their work is published in the January 2013 issue of the Journal of the American Chemical Society.
Organic liquid electrolytes are incompatible with lithium electrodes due to safety and cyclability concerns. Currently, lithium-ion batteries use a graphite anode with lithium integrated into it. These batteries cannot use pure lithium metal because during operation the metal will form wires, called dendrites, that reach across the electrolyte from one electrode to the other and short out the cell.
As principle investigator Dr. Chengdu Liang explained to Charged, liquid electrolytes – typically organic solvents – are usually highly volatile and flammable. By replacing these liquids with solids, the risk of fire is reduced both because the volatile/combustible component is gone and more importantly, because the solid electrolyte serves as a barrier to dendrite formation. This removes the need for a carbon anode and leads to a large improvement in energy density by permitting large-sized lithium anode batteries, which in the past were precluded for safety reasons. A 5 V electrochemical window, meanwhile, puts this configuration on par with, if not ahead of, most liquid electrolyte systems. The energy density is proportional to the square of the electrochemical window, so double the voltage means you can store 4 times as much energy. “Using a solid electrolyte inside [a] battery has been a dream for quite a while,” said Liang.
Engineering Details:
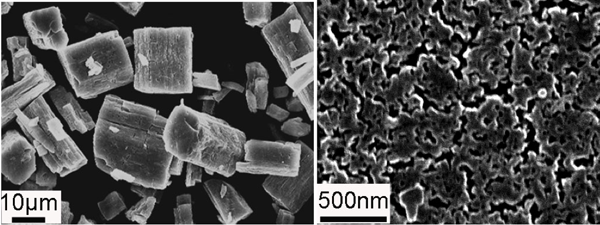
Up to this point, solid electrolytes have shown limited promise as their ionic conductivities have been orders of magnitude less than their liquid counterparts. Others still are not compatible with the lithium used in electrodes. To tackle this dilemma, the Oak Ridge team eschewed developing a new exotic electrolyte composition, in favor of choosing to manipulate the nanostructure of an existing lithium thiophosphate electrolyte, with impressive results. The electrolyte the authors studied, Li3PS4, has a γ-phase that is stable at room temperature but that phase has a low room temperature ionic conductivity, i.e. 3 X 10-7 S cm-1. However, upon heating to 195°C, this phase transforms into a β-phase that has a relatively high ionic conductivity but reverts back to the less ionically conductive γ-phase after falling below 195°C.
As it turns out, however, the devil is in the details. By adding tetrahydrofuran to the reactants used to synthesize Li3PS4 and then heating the reaction product to 80°C, amorphous Li3PS4 is produced. Heated a bit further to 140°C, something strange happens. The amorphous Li3PS4 crystallizes into a nanoporous variant of β-Li3PS4 that is stable between 25°C and 350°C – in other words, at room temperature. This nanoporous Li3PS4 was shown to have an ionic conductivity three orders of magnitude greater than normally synthesized β-Li3PS4, implying that a mechanism unique to the nanoporous β-Li3PS4 serves to enhance ionic conductivity. The authors infer that surface conductivity is the main culprit behind the enhanced ionic conductivity.
Asked whether future designers will adopt the Oak Ridge team’s nanoporous framework, Liang replied, “I think we are making progress toward that. It may not be exactly this material.” He believes that future high energy batteries will use solid electrolytes, and cites safety as a main technology driver.
Image: Oak Ridge National Laboratory
Source: Journal of the American Chemical Society
Source: Journal of the American Chemical Society