Cambridge scientists have developed a working demonstrator of a lithium-oxygen battery that is more than 90% efficient, and can be recharged more than 2,000 times.
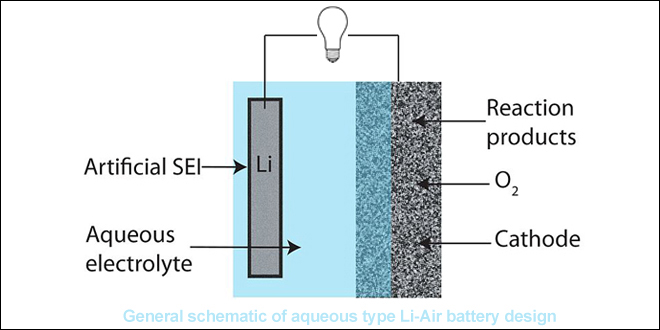
Lithium-oxygen, or lithium-air, has been touted as the ultimate battery technology, because its theoretical energy density is ten times that of lithium-ion solutions.
In “Cycling Li-O2 Batteries via LiOH Formation and Decomposition,” reported in the journal Science, the researchers demonstrated how some of the obstacles to a practical lithium-air battery may be overcome.
Their demonstrator relies on a highly porous “fluffy” carbon electrode made from graphene, and additives that alter the chemical reactions in the battery, making it more stable and more efficient.
“What we’ve achieved is a significant advance for this technology and suggests whole new areas for research – we haven’t solved all the problems inherent to this chemistry, but our results do show routes forward towards a practical device,” said Professor Clare Grey, the paper’s senior author.
The Cambridge battery uses a very different chemistry than earlier attempts at a non-aqueous lithium-air battery, relying on lithium hydroxide (LiOH) instead of lithium peroxide (Li2O2). By adding water and using lithium iodide as a “mediator,” the researchers were able to inhibit some of the unwanted chemical reactions that can cause problems, making the battery more stable after multiple charge and discharge cycles.
Other issues remain, however. The metal electrode is still prone to forming spindly lithium metal fibers known as dendrites, and the cell can only be cycled in pure oxygen.
“There’s still a lot of work to do,” said Liu. “But what we’ve seen here suggests that there are ways to solve these problems – maybe we’ve just got to look at things a little differently.”
The researchers caution that a practical lithium-air battery remains at least a decade away.
UPDATE: New papers cast doubt on lithium-air breakthrough
Source: University of Cambridge