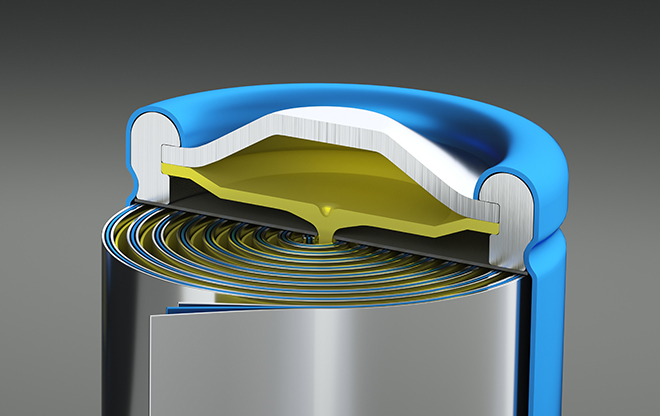
Researchers at MIT have come up with a new pulsed laser deposition technique to make thinner lithium electrolytes using less heat, which they say promises faster charging and potentially higher-voltage solid-state lithium-ion batteries.
Key to the new technique for processing the solid-state battery electrolyte is alternating layers of the active electrolyte lithium garnet component (LLZO) with layers of lithium nitride (Li3N). First, these layers are built up like a wafer cookie using a pulsed laser deposition process at about 300° C. Then they are heated to 660° C and slowly cooled, a process known as annealing.
During the annealing process, nearly all of the nitrogen atoms burn off into the atmosphere and the lithium atoms from the original nitride layers fuse into the lithium garnet, forming a single lithium-rich, ceramic thin film. The extra lithium content in the garnet film allows the material to retain the cubic structure needed for positively charged lithium ions (cations) to move quickly through the electrolyte.
The findings were reported in a Nature Energy paper published online by MIT Associate Professor Jennifer L. M. Rupp and her students.


Although other methods to produce lithium-rich ceramic materials on larger pellets or tapes, heated using a process called sintering, can yield a dense microstructure that retains a high lithium concentration, they require higher heat and result in bulkier material. The new technique pioneered by Rupp and her students produces a thin film that is about 330 nanometers thick.
“Having a thin film structure instead of a thick ceramic is attractive for battery electrolyte in general, because it allows you to have more volume in the electrodes, where you want to have the active storage capacity. So the holy grail is be thin and be fast,” says Rupp.
The new reseatch shows that during the annealing process, lithium garnet reaches the desired highly-conducting cubic phase after annealing at 660° C, much lower than the 1,050° C needed to achieve it with prior sintering methods using pellets or tapes.
“One of the greatest challenges facing the realization of solid-state batteries lies in the ability to fabricate such devices. It is tough to bring the manufacturing costs down to meet commercial targets that are competitive with today’s liquid-electrolyte-based lithium-ion batteries, and one of the main reasons is the need to use high temperatures to process the ceramic solid electrolytes,” says Professor Peter Bruce, the Chair of the Department of Materials at Oxford University, who was not involved in this research.
“This important paper reports a novel and imaginative approach to addressing this problem by reducing the processing temperature of garnet-based solid-state batteries by more than half – that is, by hundreds of degrees,” Bruce adds.
After demonstrating the processing and high conductivity of the lithium garnet electrode, the next step will be to test the material in an actual battery to explore how the material reacts with a battery cathode and how stable it is. “There is still a lot to come,” Rupp predicts.
Source: MIT News