A team of scientists that includes researchers at the DOE’s Brookhaven National Lab and SLAC National Accelerator Lab has identified the causes of degradation in nickel-rich materials for lithium-ion battery cathodes, as well as possible remedies.
Researchers at Brookhaven are part of a DOE-sponsored consortium called Battery500, a group that is working to triple the energy density of EV batteries. One of the consortium’s goals is to optimize a class of cathode materials called nickel-rich layered materials.
“Layered materials are very attractive because they are relatively easy to synthesize, but also because they have high capacity and energy density,” said Brookhaven chemist Enyuan Hu, an author of the paper.
Lithium cobalt oxide is a layered material which has been used as the cathode for lithium-ion batteries for many years, but cobalt’s cost and toxicity are barriers to the material’s use in larger systems.
“We chose a nickel-rich layered material because nickel is less expensive and toxic than cobalt,” Hu said. “However, the problem is that nickel-rich layered materials start to degrade after multiple charge-discharge cycles in a battery. Our goal is to pinpoint the cause of this degradation and provide possible solutions.”
Degradation in nickel-rich materials is mainly due to capacity fading – a reduction in the battery’s charge-discharge capacity after use.
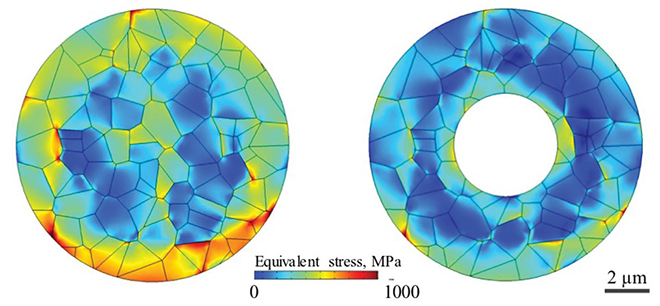

This figure compares the stress levels in a solid particle (left) to a hollow particle (right). Blue represents lower stress levels while red represents higher stress levels. Image courtesy Purdue University.
“This is a very complex material. Its properties can change at different length scales during cycling,” Hu said. “We needed to understand how the material’s structure changed during the charge-discharge process both physically – on the atomic scale up – and chemically, which involved multiple elements: nickel, cobalt, manganese, oxygen, and lithium.”
“At every length scale in this material, from angstroms to nanometers and to micrometers, something is happening during the battery’s charge-discharge process,” said co-author Eli Stavitski. “We used a technique called x-ray absorption spectroscopy [XAS] to reveal an atomic picture of the environment around the active metal ions in the material.”
Results from the XAS experimentsled the researchers to conclude that the material had a robust structure that did not release oxygen from the bulk, a finding not in line with previous beliefs. Instead, the researchers identified that the strain and local disorder was mostly associated with nickel.
Hu said, “The major conclusion we drew from this experiment was that there were considerable inhomogeneities in the oxidation states of the nickel atoms throughout the particle. Some nickel within the particle maintained an oxidized state, and likely deactivated, while the nickel on the surface was irreversibly reduced, decreasing its efficiency.”
Source: Brookhaven National Laboratory