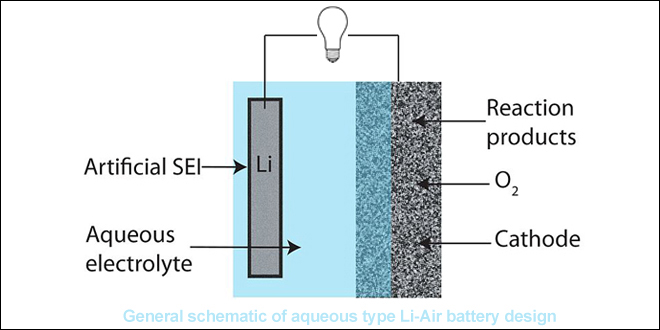
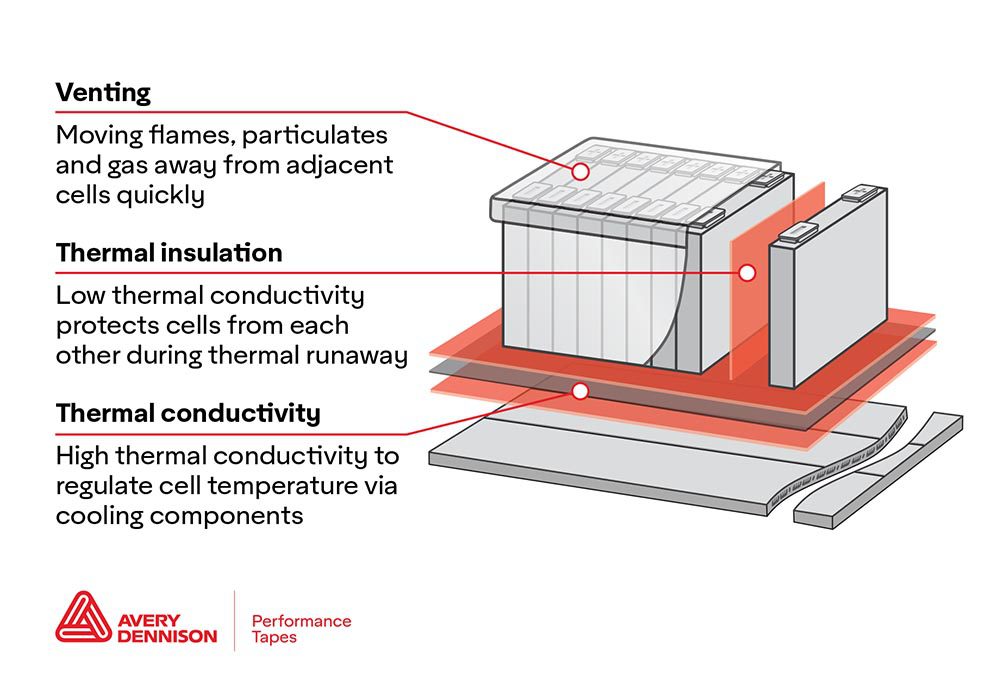
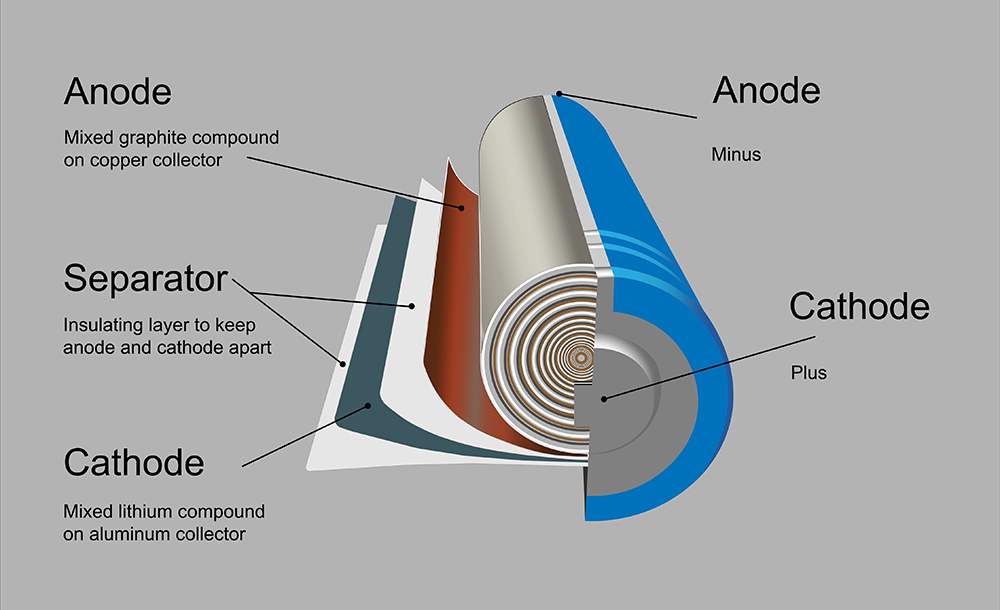
Back in October, Cambridge scientists wowed the battery world with an announcement that they had demonstrated a highly efficient and long-lasting lithium-oxygen battery. Lithium-oxygen, or lithium-air, batteries have a theoretical energy density ten times that of lithium-ion solutions, but suffer from poor cycle life.
In a paper published in the journal Science, Tao Liu and colleagues claimed to have overcome this obstacle by using a porous graphene electrode and by using lithium iodide and water as mediators.
Alas, several researchers from universities and national labs in the US, China, and Australia have now disputed the Cambridge team’s findings in two separate dissents, saying that the original results could not be replicated. The dissenters found that lithium iodide does not perform the function that the earlier paper claimed.
“Based on a simple thermodynamic analysis, we show that iodide-mediated electrochemical decomposition of lithium hydroxide (LiOH) likely occurs through a different mechanism than that proposed by Liu et al,” writes Carnegie Mellon Professor Venkatasubramanian Viswanathan in one of the dissenting papers. “It is therefore possible that the system described in Liu et al may not form the basis for a rechargeable lithium-oxygen battery.”
Another paper, from a team led by Yue Shen, reports similar findings: “We argue that LiOH cannot be oxidized by triiodide. The charge capacity is from the oxidation of I- instead of LiOH. The limited-capacity cycling test is misleading when the electrolyte contributes considerable parasitic reaction capacity.”
“The breakthrough is not a breakthrough, and we are in a sense no further along in lithium-air than we were,” Viswanathan told Quartz.
Source: Quartz