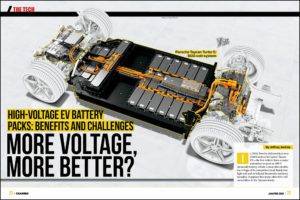
Engineers from the University of Pennsylvania and the University of Florida have recently made a breakthrough in the development of solid polymer electrolytes (SPEs). Current liquid electrolytes require thick, heavy housings in order to prevent leaks and fires from punctures, increasing their bulk and weight. SPEs solve many of these problems, but until recently, they were not well understood. After an in-depth study, the universities have created polymers that they say are thinner, lighter, and safer than other alternatives. The new materials also allow faster recharging, due to the new design which increases proton flow across the polymer.
A traditional battery has an electrolyte which allows protons to be transferred between the anode and cathode of a battery. To prevent electrons from bypassing their circuit, a barrier is used between terminals. When used with water, an SPE can function as an electrolyte-barrier combination, as it is permeable to protons but not electrons.
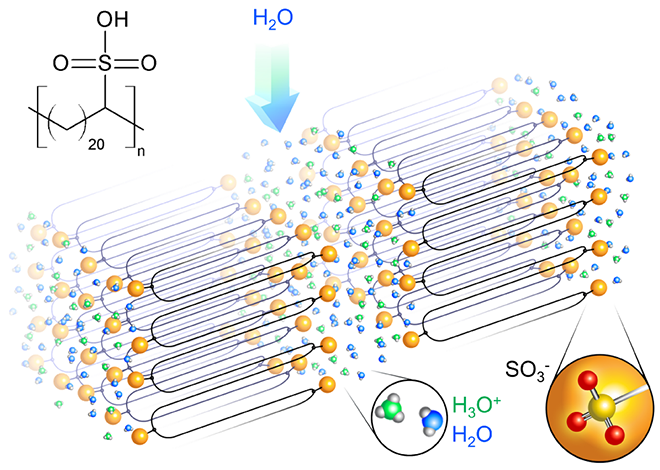
One popular SPE, Nafion, is a fluorinated polymer with several branches of sulfonic acid groups. These acid groups attract water and allow protons to move across the polymer. Unfortunately, the molecular structure of the polymer is highly irregular, which means the protons must make an arduous trek between terminals. The new study has created a way to create regular, layered channels which double their proton flow rate. “It’s like superhighways versus the country roads of Provence,” says study leader Karen I. Winey. A battery created with the new material is thinner and safer than most liquid systems. Also, the increased proton transfer rate means charging will be much faster than other SPEs. This study is one of many approaches to solving the shortcomings of liquid electrolytes and current SPEs.
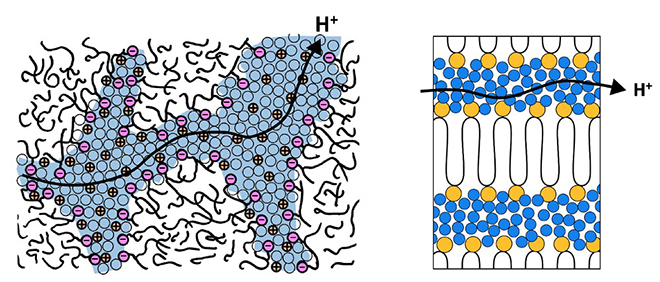
“Precision synthesis has been one of the grand challenges in polymer science, and this remarkable work demonstrates how it can open doors to novel materials of great promise,” says Linda Sapochak, Director of the National Science Foundation’s Division of Materials Research.
Source: Medium