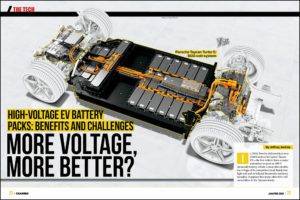
Scientists at the DOE’s Lawrence Berkeley National Laboratory have developed a novel electrolyte that addresses many of the problems of solid electrolytes by combining the two primary types – polymer and glass.
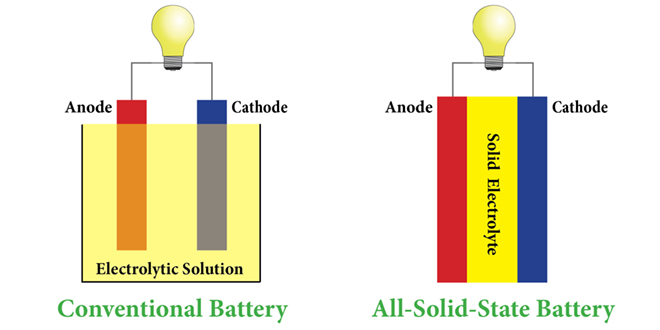
There are two kinds of solid electrolytes – polymer and glass or ceramic – and each has its own set of challenges. Polymer electrolytes don’t conduct well at room temperature, whereas ceramic electrolytes require a great deal of pressure to maintain contact with the electrodes. “It needs something like 1 ton over every square centimeter, so you need a big truck sitting on the battery as it cycles,” said senior author Nitash Balsara (also one of the co-founders of battery startup Seeo).
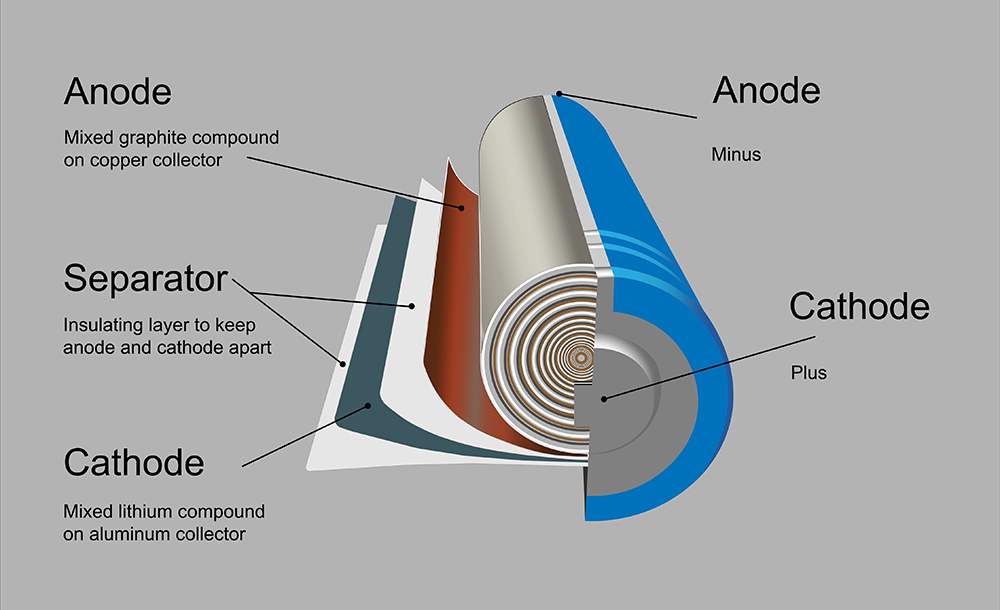
In “Compliant Glass-Polymer Hybrid Single-Ion-Conducting Electrolytes for Lithium Batteries,” published in the Proceedings of the National Academy of Sciences, the researchers explain how they developed a glass-polymer hybrid by attaching perfluoropolyether chains to the surface of glass particles, adding salt, and then making a film.
“The electrolyte is compliant, which means it can readily deform to maintain contact with the electrode as the battery is cycled, and also has unprecedented room temperature conductivity for a solid electrolyte,” said Balsara.
Although the conductivity is 10 to 15 times lower than that of a liquid electrolyte, “it’s probably good enough for some applications,” Balsara said. “We don’t necessarily need to match a liquid electrolyte because nearly all of the current in the hybrid electrolyte is carried by the lithium ion. In conventional lithium electrolytes only 20 to 30 percent of the current is carried by the lithium ion. Nevertheless, it is likely that playing around with different glass compounds, particle size, and length and concentration of the polymer chains will result in improved conductivity.”
The researchers also demonstrated that their hybrid electrolyte should be stable with two of the most promising next-generation cathode candidates: sulfur and high-voltage cathodes such as lithium nickel manganese cobalt oxide.
“People would like to use 5-volt cathodes, but electrolytes that are stable against those 5-volt cathodes are not readily available,” Balsara said. “We have demonstrated this electrolyte is stable at 5 volts, though we have not incorporated the hybrid electrolyte in the cathode yet.”