High-nickel-content cathodes have captured the imagination of scientists, as they could enable much higher energy density. However, the high nickel content causes these cathode materials to degrade more quickly, creating cracks and stability issues as the battery cycles.
A team of researchers at the DOE’s Brookhaven National Laboratory investigated the valence gradient of cathode materials in order to understand its effect on battery performance. Their findings, published in Nature Communications, demonstrate that the valence gradient can serve as a new approach for stabilizing the structure of high-nickel-content cathodes against degradation and safety issues.
Scientists have synthesized materials made with a nickel concentration gradient—that is, the concentration of nickel gradually changes from the surface of the material to its center, or bulk. These materials have exhibited greatly enhanced stability, but scientists have not been able to determine if the concentration gradient alone was responsible for the improvements. The concentration gradient has traditionally been inseparable from another effect called the valence gradient, or a gradual change in the nickel’s oxidation state from the surface of the material to the bulk.
In the new study, chemists synthesized a novel material that isolated the valence gradient from the concentration gradient.
“We used a unique material that included a nickel valence gradient without a nickel concentration gradient,” said Brookhaven chemist Ruoqian Lin, first author of the study. “The concentration of all three transition metals in the cathode material was the same from the surface to the bulk, but the oxidation state of nickel changed. We obtained these properties by controlling the material’s atmosphere and calcination time during synthesis. With sufficient calcination time, the stronger bond strength between manganese and oxygen promotes the movement of oxygen into the material’s core while maintaining a Ni2+ oxidation state for nickel at the surface, forming the valence gradient.”
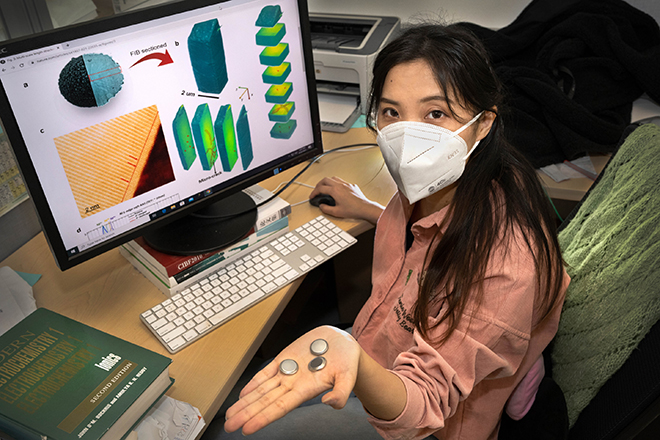
The Brookhaven researchers studied the new material’s performance using two cutting-edge experimental tools: the Hard X-ray Nanoprobe (HXN) beamline and the Full Field X-ray Imaging (FXI) beamline. By combining the capabilities of both beamlines, they were able to visualize the atomic-scale structure and chemical makeup of their sample in 3D after the battery operated over multiple cycles.
“Both beamlines have world-leading capabilities. You can’t do this research anywhere else,” said Yong Chu, leader of the imaging and microscopy program at NSLS-II and lead beamline scientist at HXN. “FXI is the fastest nanoscale beamline in the world; it’s about ten times faster than any other competitor. HXN is much slower, but it’s much more sensitive—it’s the highest resolution X-ray imaging beamline in the world.”
HXN beamline scientist Xiaojing Huang added, “At HXN, we routinely run measurements in multimodality mode, which means we collect multiple signals simultaneously. In this study, we used a fluorescence signal and a phytography signal to reconstruct a 3D model of the sample at the nanoscale. The florescence channel provided the elemental distribution, confirming the sample’s composition and uniformity. The phytography channel provided high-resolution structural information, revealing any microcracks in the sample.”
Meanwhile at FXI, “the beamline showed how the valence gradient existed in this material. And because we conducted full-frame imaging at a very high data acquisition rate, we were able to study many regions and increase the statistical reliability of the study,” Lin said.
At the CFN Electron Microscopy Facility, the researchers also used an advanced transmission electron microscope (TEM) to visualize the sample with ultra-high resolution. By combining the data collected across all of the different facilities, the researchers were able to confirm that the valence gradient played a critical role in battery performance. The valence gradient “hid” the more capacitive but less stable nickel regions in the center of the material, exposing only the more structurally sound nickel at the surface. This arrangement suppressed the formation of cracks.
“These findings give us very important guidance for future novel material synthesis and design of cathode materials, which we will apply in our studies going forward,” Lin said.
Source: Phys.org