The dry electrode coating process has the potential to enable the production of better, greener, more cost-effective batteries. It relies on advanced fluoropolymer binders with Teflon™
For a few years now, Charged has been reporting on how dry electrode coating processes have the potential to revolutionize battery production by eliminating the use of hazardous, environmentally harmful solvents. Taking the solvents out of the process can translate to big savings in cost and floor space in the factory—and the dry coating process can also enable designers to improve battery performance.
The dry electrode coating process relies on the use of special binders that can form an electrode coating without being dissolved in a solvent, such as fluoropolymer binders with Teflon™ from specialty chemical company Chemours.
To learn about the advantages of the dry coating process, and how companies are meeting the challenges involved in scaling the technology up from pilot to production scale, Charged spoke with Tejas Upasani, Global EV Technology Manager at Chemours.
Tejas Upasani: We like to call Chemours “a startup company with 200 years of history.” We spun out of DuPont in 2015, and we have leading brands in various industries, including semiconductors and automotive. Under our Advanced Performance Materials business, we have brands you might recognize, such as Teflon™, Nafion™ and Viton™.
Now we are experiencing growth in our products in a brand-new field—the dry electrode coating process—and I’m really excited to see how Chemours can support the scale-up of this new application.
In the dry process, many of the ingredients remain the same—similar active materials, similar conductive additives. What really changes is the binder.
Charged: Can you walk us through the basic advantages of the dry electrode coating process versus the traditional wet slurry-based process?
Tejas Upasani: The dry coating process is a novel way of manufacturing cathode and anode electrodes in lithium batteries.
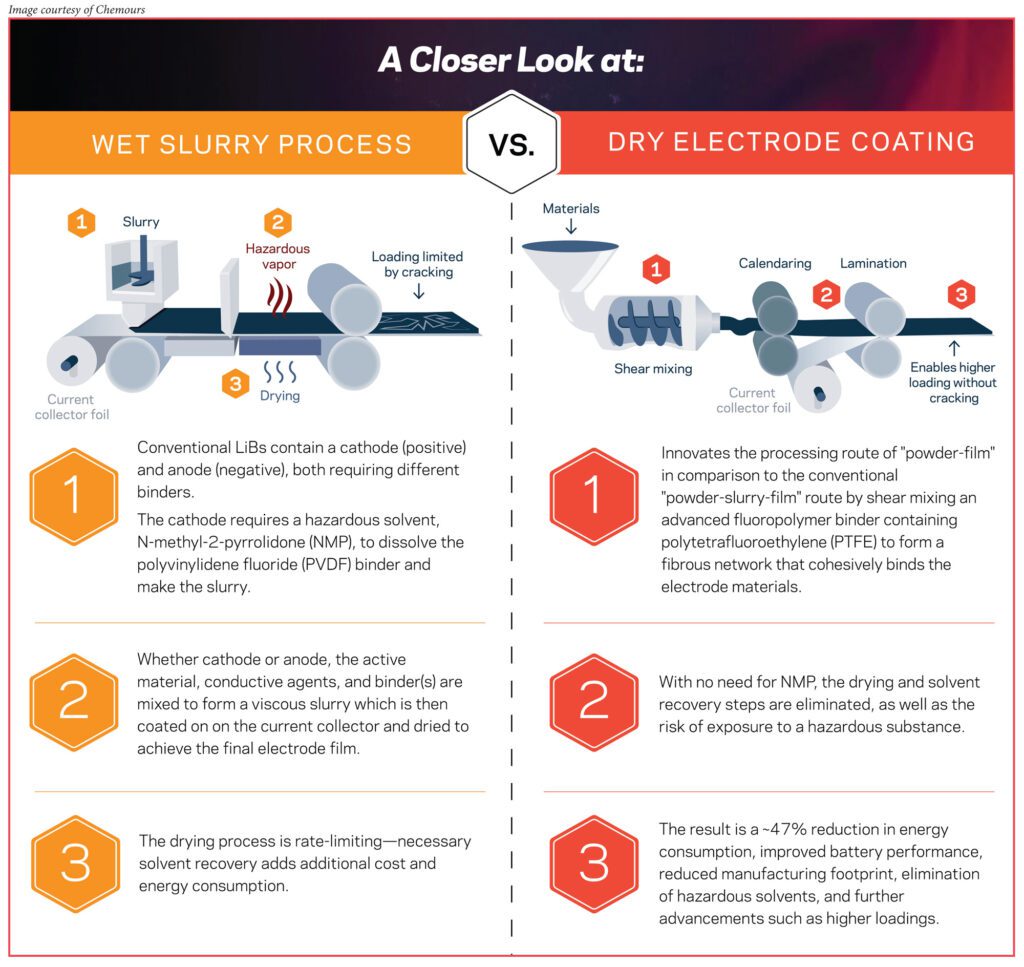
In the traditional wet slurry process, we have the active ingredients, we have the conductive additives, and we use a particular binder which needs to be dissolved in a solvent. Once all these ingredients are mixed together, we create what is called a slurry. That slurry has to be coated onto a current collector. At that point, the function of the solvent is done, so we dry off the solvent and we get a nice coating on the current collector.
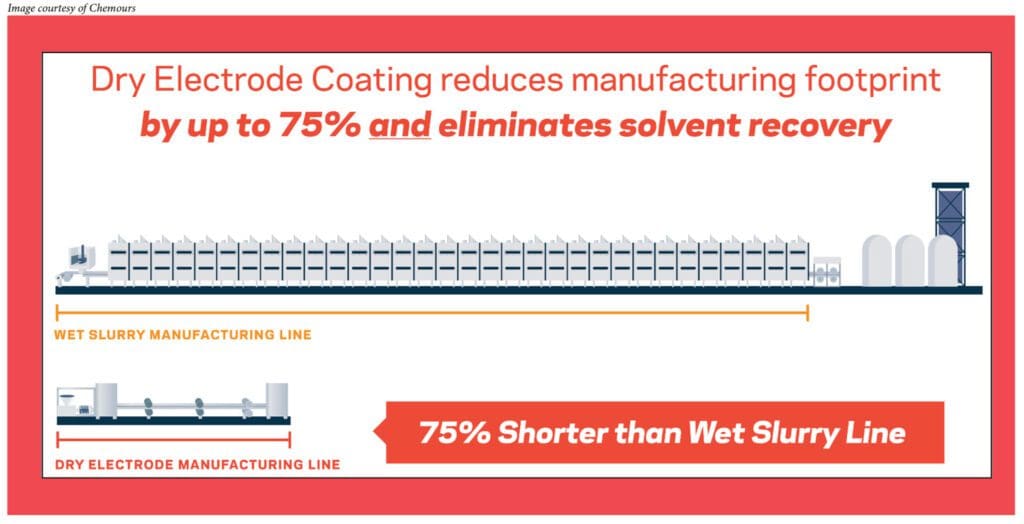
In the dry process, many of the ingredients remain the same—similar active materials, similar conductive additives. What really changes is the binder. In this case, we’ll be using advanced fluoropolymer binders with Teflon™ which, because of its unique properties, doesn’t need to be dissolved in any solvent. It can form the coating as it goes through the processing steps through a process called fibrillation, which basically forms the entire coating on the current collector.
Why is the dry process advantageous over the wet slurry process? We can look at this from three different angles.
One is that it is much more environmentally friendly. The wet slurry process uses NMP [N-Methylpyrrolidone], which is a hazardous solvent. In order to get rid of the solvent in the wet slurry process, it has to go through a series of ovens. If there is no need for the solvent, then the hazards associated with the solvent are removed.
The second part is production costs. If you look at how much space is required for the wet slurry process, by some estimates, it’s 10 times the space compared to the dry process, so there’s a tremendous amount of savings of floor space that can be achieved with the dry process.
The third aspect is that it enables better performance of the batteries. With the dry process, we can make thicker electrodes, which can help with improving power density.
Advanced fluoropolymer binders from Chemours are really at the heart and center of that process.
Charged: Is this something that could help to reduce charging times?
Tejas Upasani: It potentially could. There’s a lot of testing that is being done right now, comparing the wet slurry process and the dry process. If you are able to go to a higher loading with the dry electrode process—say, all the way to 8 or 9 milliamp-hours per square centimeter—we can see competitive or higher charging rates compared to a normal loading of the wet slurry process, which is about 3 to 4 milliamp-hours per square centimeter now. Much of this work is done at lab scale or pilot scale, but as the technology matures and we start seeing better process technologies, these can be realized in real-life scenarios as well.
Charged: Is dry electrode coating currently in production?
Tejas Upasani: We are in the early stages of the development process. Some industry players are at production scale. For example, on Battery Day in 2020, Tesla announced that they wanted to produce their 4680 cells in a dry electrode process. And on Investor Day in 2023, the company announced that they had successfully implemented commercial production of the dry electrode process. PowerCo, a subsidiary of Volkswagen, has announced that they will deploy and commercialize the dry electrode process at many different locations. LG Energy Solutions has announced similar plans.
But as it stands right now, we are seeing the entire spectrum—lab, pilot, pre-production, production—of adoption of the dry electrode process.
We think that cell manufacturers and OEMs in the next two to five years are going to be in different stages. Some are going to be at pilot scale. Others are going to advance into production scale. But as it stands right now, we are seeing the entire spectrum—lab, pilot, pre-production, production—of adoption of the dry electrode process.
Charged: Are there any major technical hurdles that we still need to get past before this can be widely adopted?
Tejas Upasani: Certainly there are hurdles. Everybody’s trying to develop the process, and they’re trying to make sure that the correct mixing and calendaring can be done in order to create a uniform structure. Some of the technical hurdles have to do with binders and the dry electrode processes enabled through understanding the fibril network of PTFE [polytetrafluoroethylene].
The use of PTFE and the resultant fibril network has been understood for decades, and we, as inventors of PTFE, have invested a lot of science behind understanding the fibril network, but it generally has been applied to industries where PTFE is the dominant component in the application. As an example, if you look at your standard plumber’s tape (Teflon™ tape), it utilizes exactly the same principle of fibrillation. That’s why you can pull it in one direction easily, but in the transverse direction, you can just break it apart.
It’s the same in this application—we’re trying to control the fibrillation through the mixing process and through the calendaring process. Chemours has invested heavily in developing various types of advanced fluoropolymer binders with PTFE. These have a range of different molecular weights and different polymer architectures, and all of these are intended to enable the proper fibrillation characteristics within the electrode process.
On the cathode side, generally PTFE is oxidatively very stable…it’s a very promising application. On the anode side there might be reductive stability challenges associated with traditional PTFE, and so using traditional PTFEs might not be the optimum solution.
Traditional PTFE may have challenges on the anode side. On the cathode side, generally PTFE is oxidatively very stable. One of the advantages is that you can go to higher voltages and it still is stable at higher-voltage applications. So, on the cathode side, it’s a very promising application.
On the anode side there might be reductive stability challenges associated with traditional PTFE, and so using traditional PTFEs might not be the optimum solution. That’s one of the reasons why we are developing a lot of different products and trying to understand the mechanism of why traditional PTFE is not stable on the anode side. And once we understand that mechanism, how do we solve that? There’s a tremendous amount of work going on internally and with our external partners as well to try and understand and solve these hurdles.
Charged: One of the challenges is adhesion. The dry material has to bond to the electrode surface, but the flat surface and lack of texture can make that difficult.
Tejas Upasani: The industry right now is using what we call carbon-coated current collectors. They have certain coatings on the current collectors, and when the dry process films are made, those get laminated onto that carbon-coated current collector.
That’s the solution that the industry has at this point, and it’s working fairly well in both anode and cathode processes. Now, if we wanted to directly laminate the film onto the current collector without any carbon coating, then that’s a little bit of a problem, and we are working on it right now.
We are looking at ways that we can alter the chemistry of the polymers themselves in order to get better adhesion to the current collectors. If we were able to directly laminate onto the current collector, why have this carbon coating?
We are looking at ways that we can alter the chemistry of the polymers themselves in order to get better adhesion to the current collectors. If we were able to directly laminate onto the current collector, why have this carbon coating? Eliminating the coating reduces the cost. I think that might come, but right now the focus is on scaling up the technology with coated current collectors.
Charged: The process needs to reduce the amount of binder and other inactive material to a similar level as that of wet coating, but this can be expensive and hard to scale up.
Tejas Upasani: Yeah. Certain cell chemistries require increasing the amount of inactive material, especially on the cathode side, whereas there are some cell chemistries where we are looking at binder loadings of less than 2%, and in some cases even less than 1%.
So, it’s already being worked on, trying to reduce the amount of inactive materials. It does require a lot of process optimization because, as you can imagine, the small amount of binder is holding up the entire powder chemistry. So, a lot of process technology, along with the material enhancements that we are doing in developing new materials and coming up with different polymer chemistries, is going to enable even further reductions of the amount of inactive materials.
Charged: Another challenge is uniformity—the dry coating mixture needs to be uniform across large areas of the battery electrodes.
Tejas Upasani: I don’t think uniformity challenges are necessarily restricted to the dry coating process. There are methods that have been developed in the wet slurry process to understand that the viscosity is right or the solids content is right, and that will help us to understand that the uniformity of the slurry is also good.
Once the mixing is done homogeneously, the beauty of the dry electrode process is that, once it is laminated onto the current collector, the coating process is done. You don’t have any movement or settling of the ingredients.
In the dry process, it’s similar, except that we are dealing with all the powders. There are analytical methods and tools that are being developed in order to verify that these powders are mixed correctly—the active materials, carbon black and binders, they need to be mixed really homogeneously. Once the mixing is done homogeneously, the beauty of the dry electrode process is that, once it is laminated onto the current collector, the coating process is done. You don’t have any movement or settling of the ingredients. In a wet slurry process, if you were to make a thick electrode, as the solvent is drying off, these ingredients may start to settle during the drying process.
Charged: So, your company would partner with the manufacturer to determine the ideal mix.
Tejas Upasani: Yes. And throughout our history, we have looked at application development. This is what we have done at Chemours for decades. We don’t want to just say to the customers, “Here’s a material, use it.” We don’t want to say that we are just a supplier. We don’t want to stop there. We want to make sure that we contribute to the success of our customers as well.
There are methods available to understand the mixing homogeneity, which are very R&D-based, and we are doing some of that work, but if someone is doing this on a production basis at a manufacturing site, they are not going to have time to take a sample, go into the R&D lab and wait for days in order to get the results. So, when we are developing these methods internally, we are trying to develop a method which is going to be in line with production characterization and analysis.
Charged: Can you tell us about your advanced fluoropolymer binders with Teflon PTFE?
Tejas Upasani: Understanding the fibrillation characteristics is really the key in enabling the dry electrode process. We have a spectrum of different products, which are available to be applied in a batch mixing process, or in a continuous mixing process. Not all of our customers are going to use the exact same way of manufacturing it, so trying to tailor our products to their needs is the key.
And given that we have tried all different sorts of chemistries for our advanced fluoropolymer binder products, it’s easier for us to understand what exactly is going to affect the fibrillation characteristics, and consequently the mechanical properties of these materials.
Also, Chemours is the only fluoropolymer manufacturer who has manufacturing sites in all three major regions—the US, Europe and Asia/Pacific. When we think about a scenario where the manufacturing is going to be scaled up to a production scale, we have the flexibility of having the products being made at different locations and supporting our customers with the same quality, the same safety standards and same standards applied to responsible manufacturing.
Charged: We’ve heard about some proposed regulations in Europe around PFAS that could impact PTFE. What impact would this have on dry electrode coating?
Tejas Upasani: I’m glad that you asked the question, because sometimes it is the elephant in the room when we are talking with our industry partners.
We at Chemours firmly believe that our fluoropolymers can be manufactured responsibly, and we are in favor of industry-wide national regulations and testing requirements, which are based on science and facts—data-driven regulations and testing methods, we are completely in favor of that.
We spend a lot of time, money and resources in identifying the sources of emissions from manufacturing fluoropolymers, and installing abatement systems in order to control those emissions. We are also engaging heavily in trying to develop alternate manufacturing technologies. All of these are steps that we are taking in order to meet the needs of potential regulation.
If we look at the EU regulations, particularly, it’s not necessarily confined to PTFE. PVDF, which is a fluoropolymer used in the wet slurry process, could also be potentially impacted by the same regulations.
Fluoropolymers in general are essential to lithium-ion batteries, and they’re essential for us to transition to a clean energy environment. So, we want to be partners in the regulation to make sure that the regulations address the concerns, and that these products are manufactured in a responsible way, and we are committed to doing both things.
This article first appeared in Issue 69: July-September 2024 – Subscribe now.