A team of researchers from the University of Maryland (UMD) and the US Army Research Laboratory (ARL) have devised what they call a “water-in-salt” electrolyte that could offer power, efficiency and longevity comparable to Li-ion batteries, but without the toxic chemicals and fire risk.
In “Water-in-salt electrolyte enables high-voltage aqueous lithium-ion chemistries,” published in the journal Science, Liumin Suo and colleagues describe a breakthrough that makes it possible to form a solid-electrolyte interphase in water-based batteries.
The ester-based solvents used in non-aqueous electrolytes are highly flammable and reactive with the charged electrodes, so battery designers spend a lot of time and money on safety management systems to prevent an incendiary encounter between the flammable electrolytes and the energy-intensive electrodes.
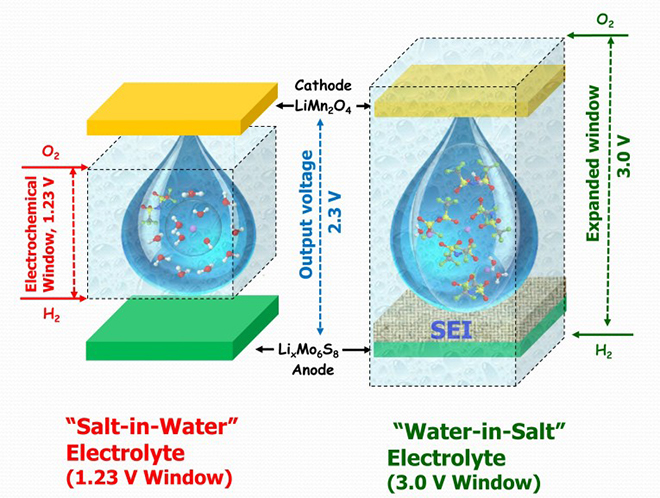
Aqueous electrolytes could resolve these concerns, but their electrochemical stability window (1.23 V) is too narrow to support most of the electrochemical couples used in Li-ion batteries.
Also, in contrast to non-aqueous electrolyte systems, kinetic protection from a solid-electrolyte interphase (SEI) has never been considered possible in aqueous media. Such interphases constitute a barrier between electrode surfaces and electrolytes, allowing ionic conduction but forbidding electronic conduction. Their presence substantially expands the usable electrochemical stability window of electrolytes.
“In the absence of interphases, aqueous Li-ion batteries are typically limited to low voltage (<1.5 V) and low energy density (<70 Wh/kg), often with rapid fading of capacity and low coulombic efficiency,” said lead author Liumin Suo. “We report the formation of such interphases in an aqueous electrolyte by manipulating the source of electrolyte decomposition during the initial charging processes.”
“Through this work we were able to increase the electrochemical window of aqueous electrolyte from less than 1.5 Volts to ~ 3.0 Volts and demonstrated an aqueous full lithium-ion cell with 2.3 Volts, showing for the first time that aqueous batteries could seriously compete in terms of power and energy density with the non-aqueous lithium-ion batteries that power our mobile, digital lifestyle,” said co-author Chunsheng Wang.
Source: University of Maryland via Green Car Congress