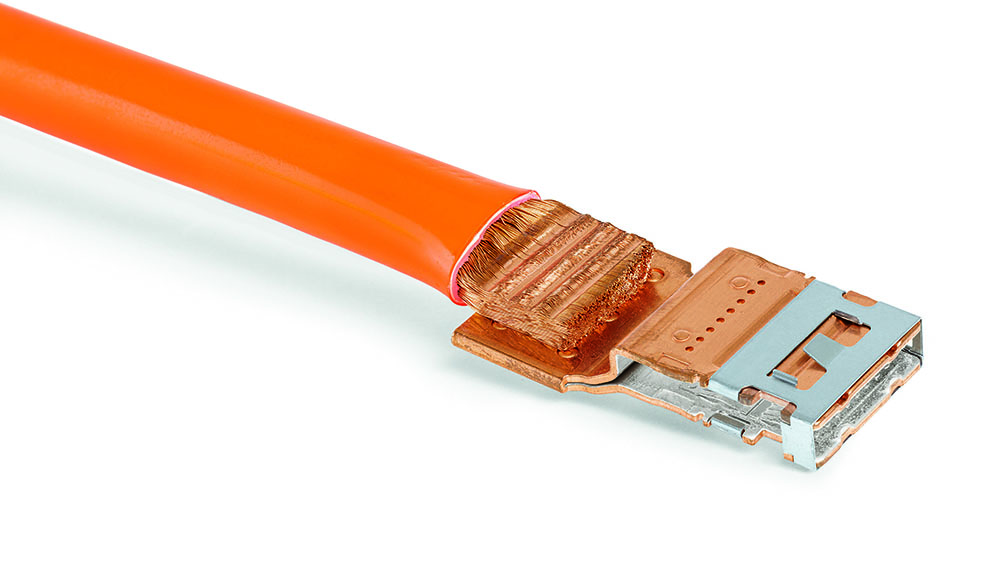
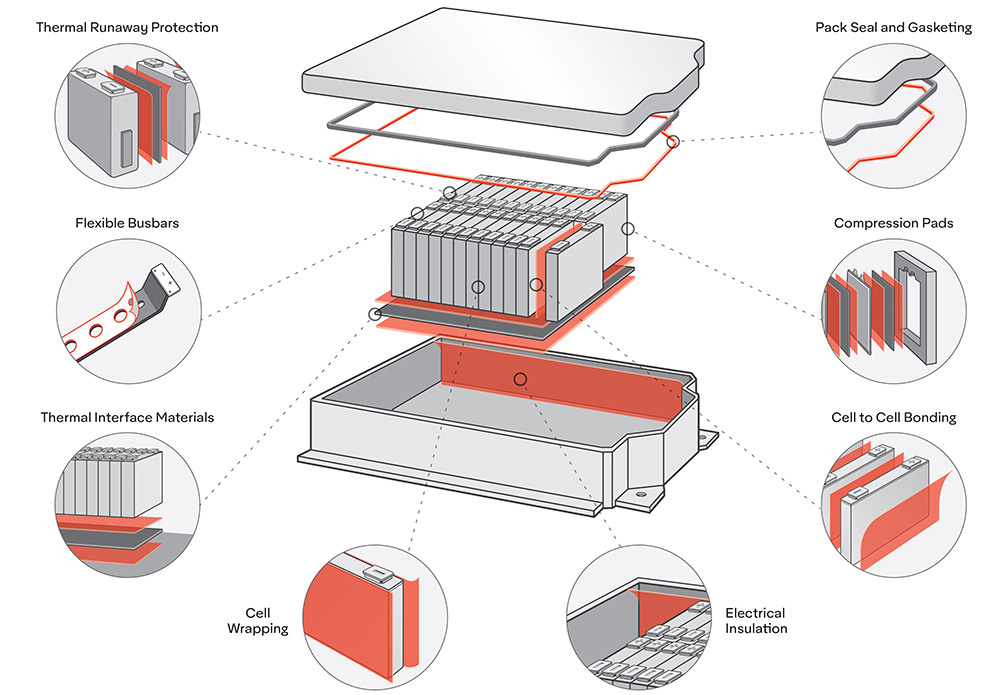
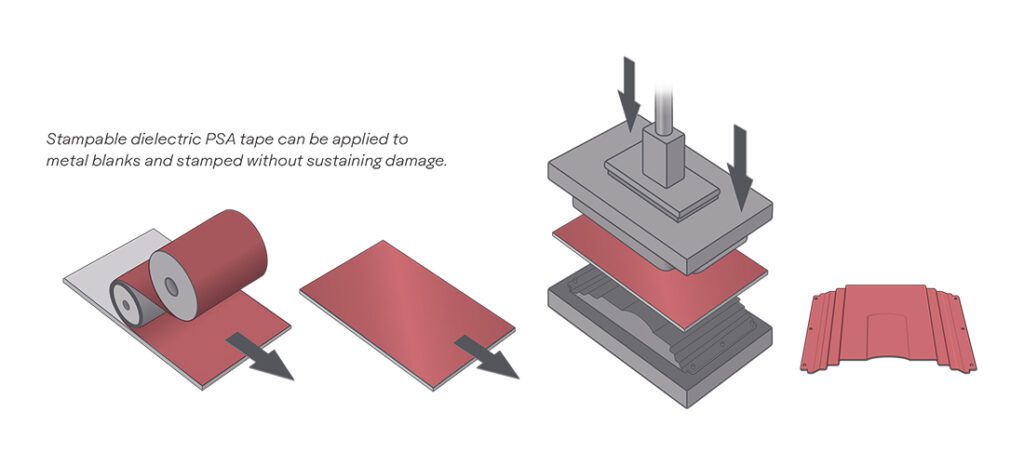
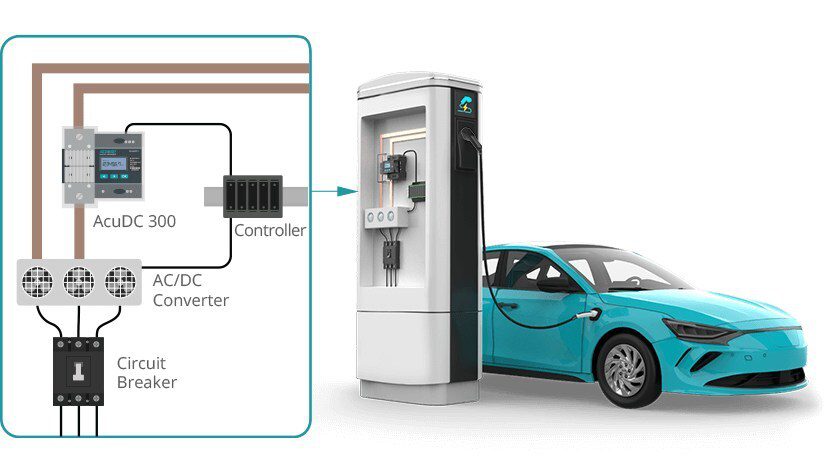
Scientists from the University of Waterloo have devised several new electrochemical processes which greatly increase the lifespan and stability of lithium-oxygen batteries. On paper, Li-O2 batteries have high energy densities and low weights. However, they also have internal reactions (involving superoxides and peroxides) that slowly corrode their carbon cathodes. The superoxide also destroys organic electrolytes in the reaction, further reducing the battery’s lifespan.
To overcome these obstacles, the researchers replaced the organic electrolyte with an inorganic molten salt and swapped the carbon cathode for a bifunctional metal oxide catalyst. They also chose to operate the battery at high temperatures (150˚ C) which produce stable Li2O instead of Li2O2 peroxide. The final result was not only more reversible but also had a coulombic efficiency near 100%. Also, a four-electron transfer was achieved, which theoretically increases energy capacity by 50%.
“We demonstrate that by increasing the operating temperature and exploiting stable inorganic electrolytes and ORR catalysts, the reversible formation of Li2O leads to a highly rechargeable Li-O2 cell with high capacity, low overpotential with the transfer of 4 e– over O2, and excellent cycling performance,” said the Waterloo researchers Xia et al.
Source: University of Waterloo via Green Car Congress