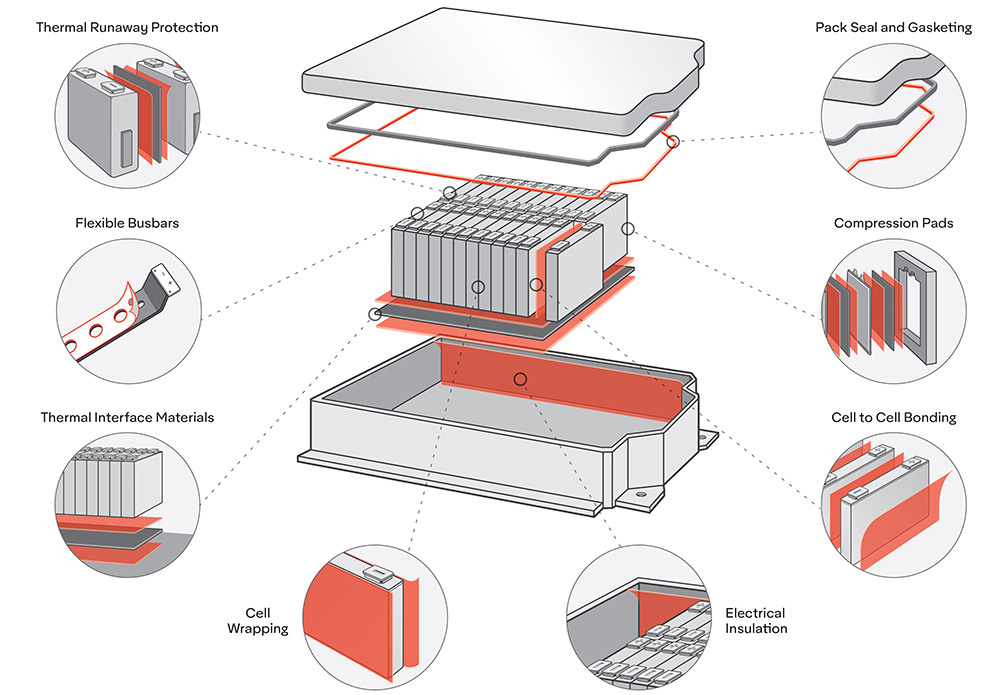
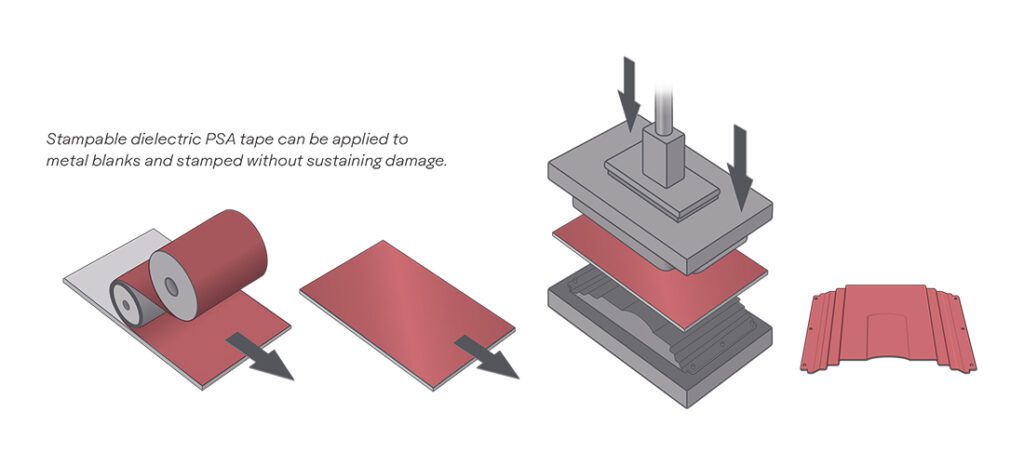
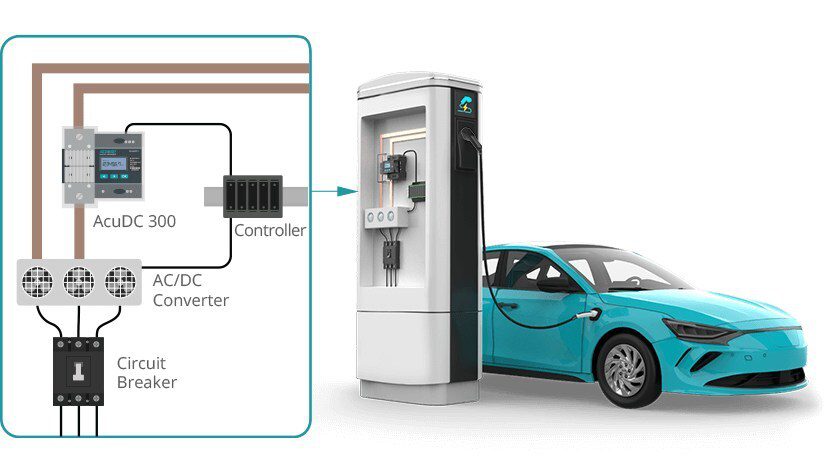
As the search for a better battery grinds on, a lot of the attention is focused on potential cathode materials. Nickel is one possibility that shows a lot of promise – its drawback is that it is unstable and tends to have destructive reactions with the electrolyte.
Now a team of scientists from the Brookhaven and Lawrence Berkeley National Laboratories and SLAC National Accelerator Laboratory say they’ve found a way to avoid this problem. In a paper published in the journal Nature Energy, the researchers explain how a battery cathode with a hierarchical structure can protect the reactive material from degradation.
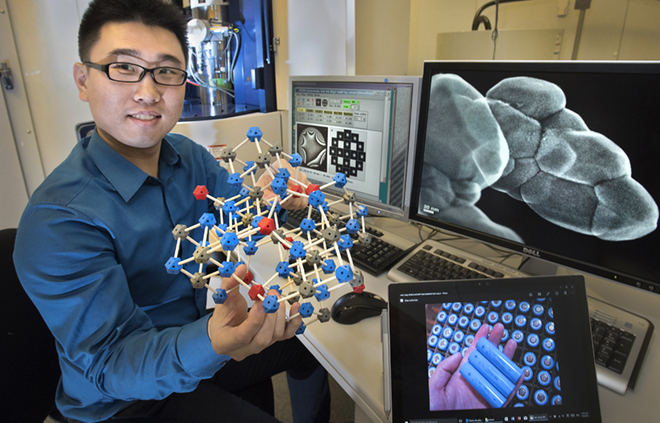
Brookhaven Lab physicist Huolin Xin described “a particle structure that has two levels of complexity where the material is assembled in a way that it protects itself from degradation.”
The scientists created a powder composed of lithium, nickel, manganese, and cobalt. Repeatedly heating and cooling the powder triggered the formation of nanosized particles that self-assembled into larger spherical, sometimes hollow, structures.
“The manganese layer forms an effective barrier, like paint on a wall, protecting the inner structure of the nickel-rich ‘bricks’ from the electrolyte,” said Huolin Xin.
But how were the lithium ions still able to enter the material to react with the nickel? The scientists found that the particles had facets like the cut edges of a crystal, which allowed them to pack tightly together to form coherent interfaces. But there was a slight misfit between the two surfaces, with the atoms on one side of the interface being slightly offset.
“The packing of atoms at the interfaces between the tiny particles is slightly less dense than the perfect lattice within each individual particle, so these interfaces basically make a highway for lithium ions to go in and out,” Xin said.
Sources: Brookhaven National Laboratory, SLAC National Accelerator Laboratory