Researchers from Stanford University and the DOE’s SLAC National Accelerator Laboratory have developed a battery electrode that heals itself, a very useful trick indeed.
“Self-healing is very important for the survival and long lifetimes of animals and plants,” said Chao Wang, one of two principal authors of the paper, which appeared in the latest issue of Nature Chemistry. “We want to incorporate this feature into lithium-ion batteries so they will have a long lifetime as well.”
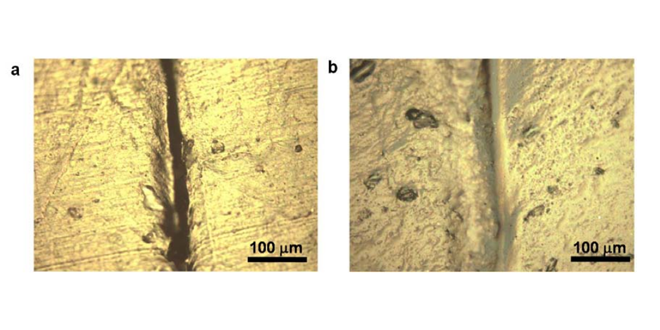
In the quest for the Grail of higher energy density, one of the most promising electrode materials is silicon, because it has a high capacity for soaking up and then releasing lithium ions. However, silicon electrodes swell and shrink as the battery charges and discharges, and the brittle material soon cracks and falls apart. The research team used a stretchy polymer that coats the electrode, binds it together and spontaneously heals tiny cracks that develop during battery operation.
“We found that silicon electrodes lasted 10 times longer when coated with the self-healing polymer, which repaired any cracks within just a few hours,” said Professor Zhenan Bao.
“Their capacity for storing energy is in the practical range now, but we would certainly like to push that,” said Professor Yi Cui. The electrodes worked for about 100 charge-discharge cycles without significantly losing their energy storage capacity. “That’s still quite a way from the goal of 3,000 cycles for an electric vehicle, but the promise is there, and from all our data it looks like it’s working.”
To make the self-healing coating, scientists deliberately weakened some of the chemical bonds within polymers. The resulting material breaks easily, but the broken ends are chemically drawn to each other and quickly link up again, mimicking the process that allows biological molecules such as DNA to assemble, rearrange and break down.
Researchers have tested a number of ways to keep silicon electrodes intact, but the self-healing electrode, which is made from silicon microparticles that are widely used in the semiconductor and solar cell industry, is the first solution that seems to offer a practical road forward, Cui said.
Image Credit: C. Wang et al, Nature Chemistry
Source: Science Daily