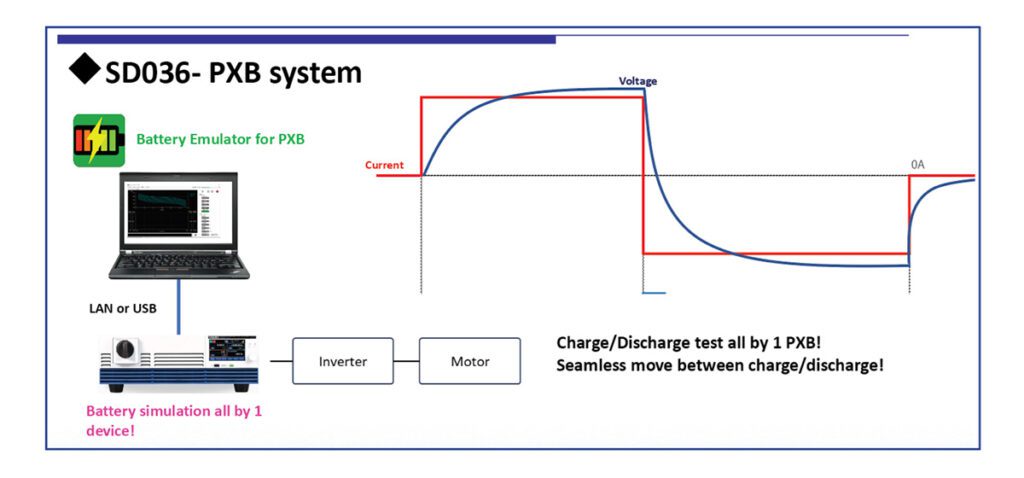
Demand for lithium is on the rise, and the rush is on for new sources of the light white stuff. Researchers in Finland are developing new methods to extract lithium from natural brine that promise more efficiency and greater purity.
Most lithium is produced from salt lake deposits. Brine is pumped up and concentrated by evaporation in large pools under the sun, then the concentrated solution is purified and the lithium is separated.
In “Removal of calcium and magnesium from lithium brine concentrate via continuous counter-current solvent extraction,” published in Hydrometallurgy, Sami Virolainen and colleagues at the Lappeenranta University of Technology (LUT) explain how they have been using solvent extraction to purify the solution. Calcium and magnesium were separated from the concentrated lithium salt solution into an organic solution consisting mainly of kerosene.
“We were typically able to purify 99-100 per cent of calcium and also over 90 per cent of magnesium. Lithium loss only amounted to 3-5 percent. In traditional methods, the purification outcome is either weaker, the lithium loss is more substantial, or both,” explains Virolainen. “The extraction process we use is more expensive than regular precipitation but separation is more efficient and easier. This simplifies the overall process, which also makes it an economically sensible alternative.”
Solvent extraction is also suitable for the separation of lithium and other metals from electronic waste, which could be a handy technology as supplies grow tight. “As the demand grows, recycling of products containing lithium and the use of new alternative sources for raw material must be increased,” Virolainen says.
The researchers demonstrated the new separation process on a pilot scale, with flow rates from one liter to five liters per hour. “On the industrial scale, we are talking about a cube or dozens of cubes per hour. However, the process has been constructed similarly as it would be in the industry, i.e. constant streams go in and come out and the number of processing phases is the same as in an industrially conducted extraction.”
Source: Lappeenranta University of Technology via Green Car Congress
Image: psyberartist – CC BY 2.0)