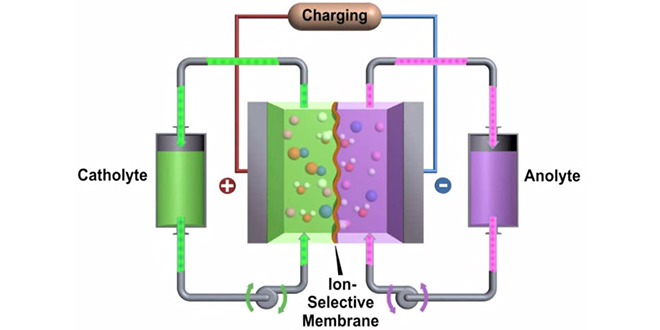
Researchers at Pacific Northwest National Laboratory (PNNL) have developed a new zinc-polyiodide redox flow battery that could offer more than two times the energy density of existing designs.
Flow batteries, which use ionized liquids stored in external tanks in place of traditional electrolytes, have garnered a lot of publicity lately, as the storage medium of the spectacular Quant sports cars displayed at the recent Geneva Auto Salon. PNNL’s flow battery, however, is more likely to find its application as stationary storage to support the power grid.
In a paper published in Nature Communications, the PNNL team describes how they created a small battery on a lab benchtop, using two glass vials containing a black zinc-polyiodide liquid and a clear zinc-iodide liquid. The 12-watt-hour demonstration battery put out far more energy for its size than today’s most commonly used flow batteries.
In lab tests, the demonstration battery discharged 167 Wh/l of electrolyte. In comparison, zinc-bromide flow batteries generate about 70 Wh/l, vanadium flow batteries can create between 15 and 25 Wh/l, and standard lithium iron phosphate batteries could put out about 233 Wh/l. The team calculated that their new battery theoretically could discharge up to 322 Wh/l, if more chemicals were dissolved in the electrolyte.
Lithium-ion batteries have more energy density than flow batteries, but their packaging can make them prone to overheating and catching fire. Flow batteries, on the other hand, store their active chemicals separately until power is needed, reducing safety concerns.
PNNL’s zinc-polyiodide battery is also safer than other flow batteries, because its electrolyte isn’t acidic. It’s nearly impossible for the water-based electrolyte to catch fire, and it doesn’t require expensive materials that are needed to withstand the corrosive nature of other flow batteries. PNNL’s new flow battery can also operate in extreme climates. The electrolyte allows it to work well in temperatures as cold as -4˚ F and as warm as +122˚ F.
“With improved energy density and inherent fire safety, flow batteries could provide long-duration energy storage for the tight confines of urban settings, where space is at a premium,” said Imre Gyuk of the DOE’s Office of Electricity Delivery and Energy Reliability, which funded this research. “This would enhance the resiliency and flexibility of the local electrical grid.”
“Another, unexpected bonus of this electrolyte’s high energy density is it could potentially expand the use of flow batteries into mobile applications such as powering trains and cars,” said the study’s corresponding author, Wei Wang.
Wei and his colleagues will continue to experiment with different alcohols and other additives and use advanced instruments to characterize how the battery’s materials respond to those additives. The team will also build a larger, 100-watt-hour model of the battery for additional testing.
Source: Pacific Northwest National Laboratory via Green Car Congress