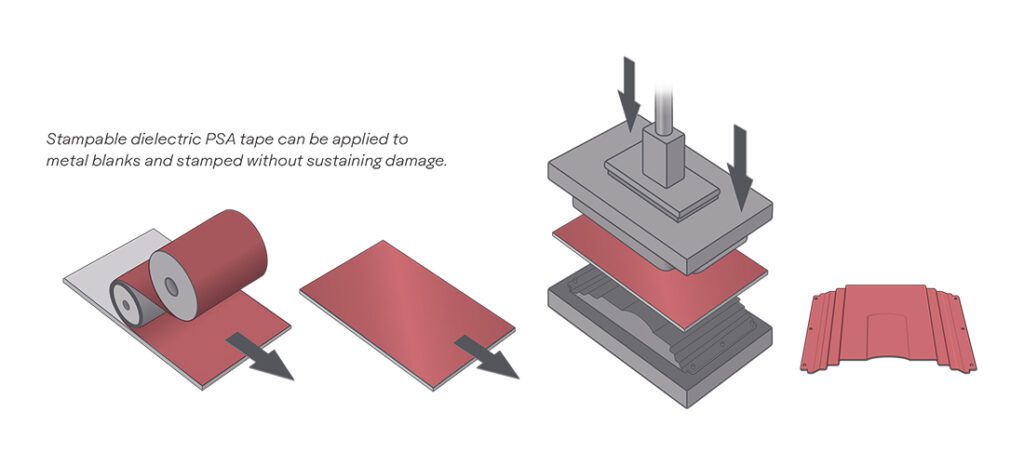
Lithium-air batteries could theoretically deliver an energy density comparable to that of gasoline. However, in most systems, the reaction pathways either involve one- or two-electron transfer, leading to lithium peroxide (Li2O2) or lithium superoxide (LiO2), respectively.
Researchers at the Illinois Institute of Technology and Argonne National Laboratory are investigating a way to create a lithium-air battery based on lithium oxide (Li2O) formation, which could deliver much higher energy density.
Lithium oxide formation involves a four-electron reaction that is more difficult to achieve than the one- and two-electron reaction processes. The researchers used a composite polymer electrolyte based on Li10GeP2S12 nanoparticles embedded in a modified polyethylene oxide polymer matrix and found that Li2O was the main product in a room-temperature solid-state lithium-air battery. The battery is rechargeable for 1,000 cycles with a low polarization gap and can operate at high rates.
“The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons,” said Argonne chemist Rachid Amine. “More electrons stored means higher energy density.”
Source: Science Journal