Lithium-sulfur batteries theoretically offer high energy density, and are widely seen as a promising next-generation energy storage system. However – you guessed it – they have several drawbacks. Among other issues, capacity tends to fade quickly.
Researchers at the DOE’s Pacific Northwest National Laboratory (PNNL) have identified one of the reasons behind this problem, and found that using LiTFSI-based instead of LiFSI-based electrolytes greatly improves stability.
In “Effect of the Anion Activity on the Stability of Li Metal Anodes in Lithium-Sulfur Batteries,” published in Advanced Functional Materials, study leader Dr. Ji-Guang (Jason) Zhang explains that salts used in the liquid electrolyte make a big difference.
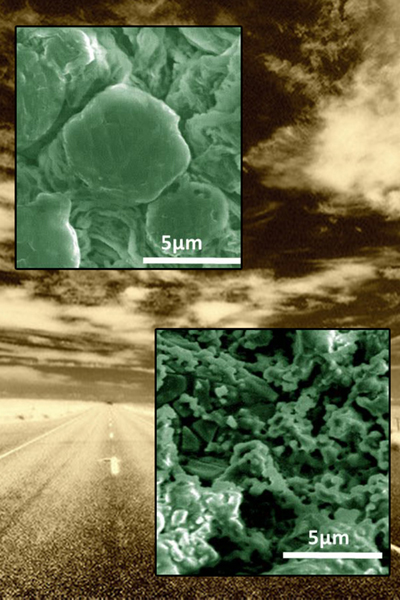
The lithium metal anode (bottom) corrodes after just 35 cycles in the LiFSI electrolyte, while the lithium anode stays relatively stable in the LiTFSI electrolyte after more than 200 cycles.
Anode images courtesy of PNNL; background image courtesy of Scott Butner
A test battery using the salt LiTFSI held most of its charge for more than 200 uses. The LiTFSI helps bind up lithium atoms and sulfur on the electrode but quickly releases them. In contrast, LiFSI ties up the lithium and sulfur but doesn’t release it. The result is an electrode that degrades after a few dozen uses.
The team found that with a LiTFSI-based electrolyte, the electrode’s lithium atoms became bound up with sulfur, forming lithium sulfide (LiSx). With LiFSI, lithium sulfate (LiSOx) formed instead. The lithium sulfide easily broke apart to release the lithium, but the lithium sulfate was hard to separate.
This fundamental difference in the bond strength of the salt anions in the presence of polysulfide species leads to a large difference in the stability of the anode-electrolyte interface, and thus in battery performance. The researchers concluded that anion selection is one of the key parameters in the search for new electrolytes for Li-S batteries.
“By conducting a macroscopic compositional analysis combined with simulations, we can see which bonds are easily broken and what will happen from there,” said Dr. Zhang. “This process lets us identify the electrolytes’ behavior, guides us to design a better electrolyte, and improve the cycle life of lithium-sulfur batteries.”
Source: Pacific Northwest National Laboratory via Green Car Congress