Researchers from the University of Maryland and the DOE’s Brookhaven National Laboratory have developed a cathode material containing iron trifluoride (FeF3) that they say could triple the energy density of Li-ion batteries. Their research was published in Nature Communications.
“Compared to the large capacity of the commercial graphite anodes used in lithium-ion batteries, the capacity of the cathodes is far more limited,” said Xiulin Fan, one of the lead authors of the paper. “Cathode materials are always the bottleneck for further improving energy density.”
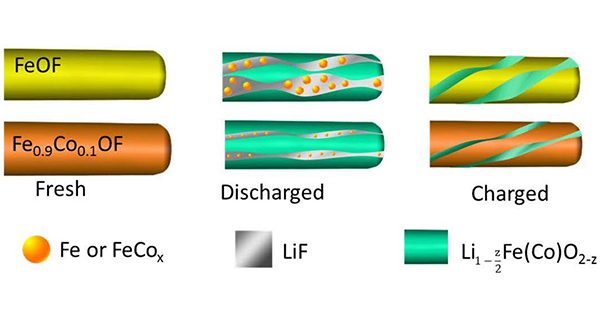
Scientists at UMD synthesized a new cathode material, an engineered form of iron trifluoride (FeF3). Researchers have long been interested in compounds such as FeF3 that offer inherently higher capacities than traditional cathode materials.
“The materials normally used in lithium-ion batteries are based on intercalation chemistry,” said fellow lead author Enyuan Hu. “This type of chemical reaction is very efficient; however, it only transfers a single electron, so the cathode capacity is limited. Some compounds like FeF3 are capable of transferring multiple electrons through a more complex reaction mechanism, called a conversion reaction.”
Brookhaven scientists are shown at the Center for Functional Nanomaterials. Pictured from left to right are: (top row) Jianming Bai, Seongmin Bak, and Sooyeon Hwang; (bottom row) Dong Su and Enyuan Hu.
Despite FeF3’s potential to increase cathode capacity, the compound has not historically worked well in lithium-ion batteries due to three limitations: poor energy efficiency (hysteresis), a slow reaction rate, and side reactions that can cause poor cycling life. To overcome these problems, the researchers added cobalt and oxygen atoms to FeF3 nanorods through a process called chemical substitution. This allowed the scientists to manipulate the reaction pathway and make it more reversible.
“When lithium ions are inserted into FeF3, the material is converted to iron and lithium fluoride,” said co-author Sooyeon Hwang. “However, the reaction is not fully reversible. After substituting with cobalt and oxygen, the main framework of the cathode material is better maintained and the reaction becomes more reversible.”
Source: Brookhaven National Laboratory