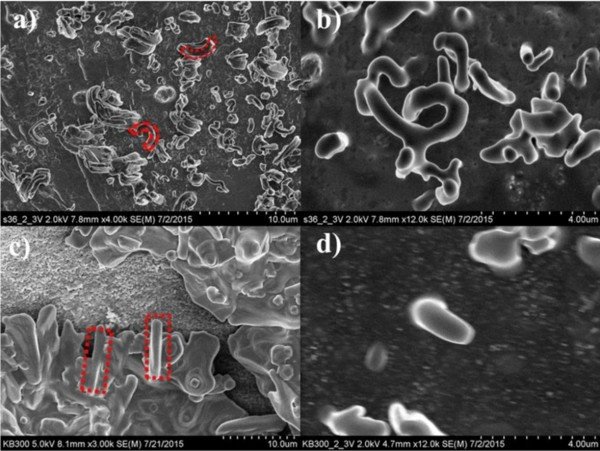
Images from Field Emission Scanning Electron Microscope (FESEM) show catalyzed cells exhibiting longer cycle life than uncatalyzed cells under similar cycling conditions.
Credit: Ates, M. N., et al. (2016)
Lithium-air batteries, which have the highest theoretical energy density of all known battery devices, could revolutionize the energy economy. However, there are several technical challenges to be met before Li-air is commercially viable.
K.M. Abraham, who published the first work on non-aqueous lithium-air batteries in 1996, is the co-author of a new study that takes a fundamental step forward in advancing Li-air through the development of a mixed metal catalyst that could lead to more efficient electrode reactions.
“Li-air is now highly researched, but there are still barriers that we have to overcome before it can go into full-fledged practical use,” Abraham says. “I think there will be some limited use in the near future in specialty applications, but it will be a while before it becomes a fully used technology.”
In “In Situ Formed Layered-Layered Metal Oxide as Bifunctional Catalyst for Li-Air Batteries,” published in the Journal of The Electrochemical Society, Abraham and his colleagues describe a cathode catalyst composed of three transition metals (manganese, nickel, and cobalt), which can create the right oxidation state during the battery cycling to enable both the catalysis of the charge and the discharge reaction.
The manganese allows for the catalysis of the oxygen reduction reaction while the cobalt catalyzes the charge reaction of the battery.
“This offers opportunities for future research to develop similar materials to optimize the catalysis of the Li-air battery using one material that will combine the functions of these mixed metal oxides,” Abraham says.
Because transition metal oxides are also developed as cathodes for Li-ion batteries, Abraham sees a possibility to use the two battery types together. “Since these catalyst materials are also being developed for Li-ion battery cathodes, it might be a way to use the electrodes from spent Li-ion batteries as a catalyst in Li-air batteries,” he says.
Source: The Electrochemical Society