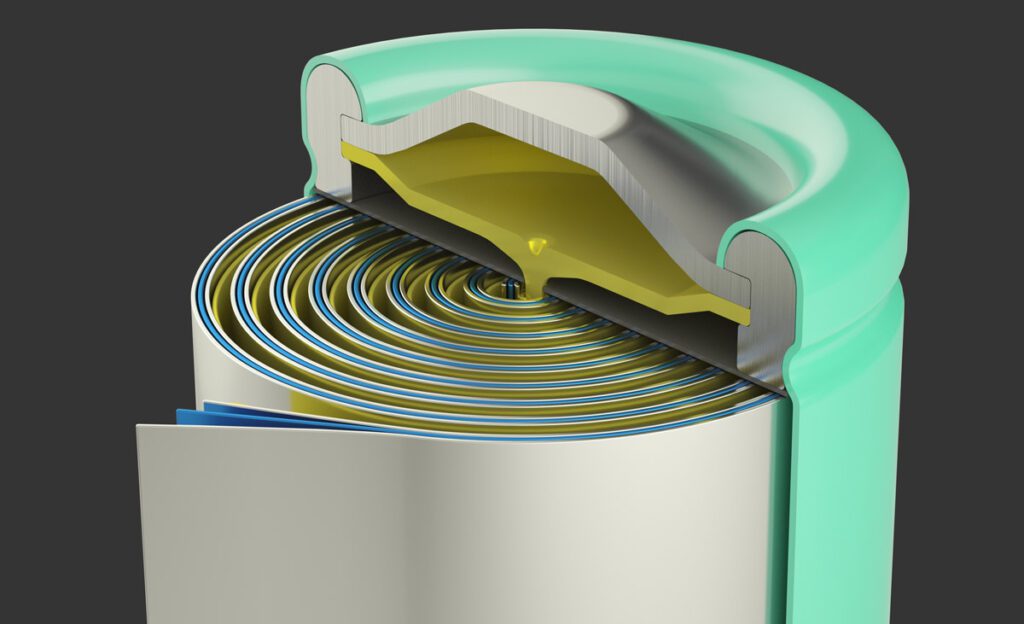
Replacing a liquid electrolyte with a solid electrolyte could offer major advantages for both safety and energy storage capacity, but so far, attempts to develop a practical solid-state cell have run into major obstacles.
Liquid electrolytes can be flammable, and are also prone to the formation of dendrites -thin, fingerlike projections of metal that build up from one electrode and can eventually create a short circuit. Researchers have tried to get around these problems by using an electrolyte made out of solid materials, such as ceramics, but these materials tend to perform erratically and are prone to short circuits.
A new paper suggests that the problem may be an incorrect interpretation of how such batteries fail. In “Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes,” published in the journal Advanced Energy Materials, MIT Professor Yet-Ming Chiang and several colleagues report new findings that could open up new avenues for developing solid-state batteries.
“The formation of dendrites, leading to eventual short-circuit failures, has been the main reason that lithium-metal rechargeable batteries have not been possible,” Chiang explains. The problem of dendrite formation in lithium rechargeable batteries was first recognized in the early 1970s, “and 45 years later that problem has still not been solved. But the goal is still tantalizing.”
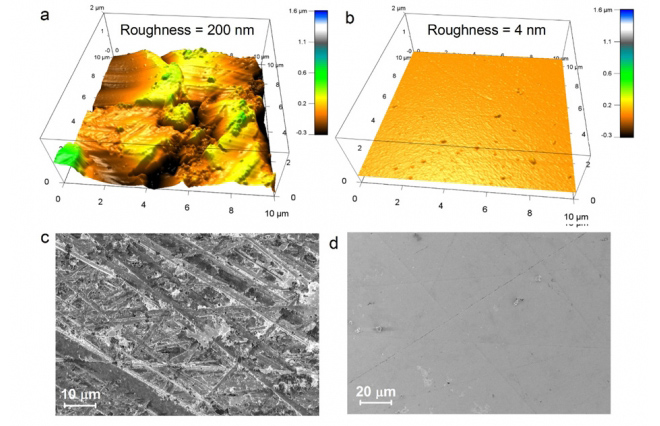
The prevailing idea is that a material’s shear modulus (firmness or squishiness) determines whether dendrites can penetrate into the electrolyte. But the new analysis showed that it’s the smoothness of the surface that matters most. Microscopic nicks and scratches on the electrolyte’s surface can provide a toehold for the metallic deposits to force their way in.
This suggests, Chiang says, that simply focusing on achieving smoother surfaces could greatly reduce the problem of dendrite formation.
These materials are “very sensitive to the number and size of surface defects, not to the bulk properties” of the material, Chiang says. “It’s the crack propagation that leads to failure. What we should be focusing on more is the quality of the surfaces, on how smooth and defect-free we can make these solid electrolyte films.”
Source: MIT