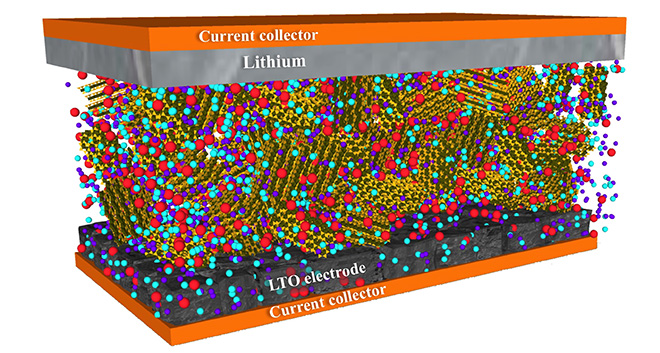
Bentonite clay is a malleable material with myriad applications, from oil drilling to agriculture to construction. Now researchers at Rice University have taken advantage of its qualities to develop a new class of Li-ion battery electrolytes that offer the structural stability of a solid and the wettability of a liquid.
In “Quasi-Solid Electrolytes for High Temperature Lithium Ion Batteries,” published in ACS Applied Materials & Interfaces, the scientists explain how they mixed a room-temperature ionic liquid and lithium salt into the clay. The liquefied salt acts as a source of lithium ions that conduct through the electrolyte to the electrodes.
The Rice researchers previously used bentonite and room-temperature ionic liquids in their work on supercapacitors. However, using the clay as an electrolyte presented a different set of challenges.

A clay-based electrolyte and separator compound. Photo by Jeff Fitlow
“Clay naturally has a lot of moisture in it, and that’s not a problem when you’re doing supercapacitors,” said lead author Kaushik Kalaga. “But a battery has to have a lithium-ion conductive species in the electrolyte to conduct lithium ions from the cathode or anode, or vice versa, when you charge and discharge. Lithium is very reactive with water, so our first challenge was to eliminate water from the clay while keeping its structure intact.”
Unlike conventional organic electrolytes, the bentonite-based brew works well at high temperatures, and it connects more easily with electrodes than most solid-state electrolytes. Test cells were able to deliver current at high temperatures over 120 charge-discharge cycles.
“It’s able to produce pretty good performance at room temperature, but it gets better at higher temperatures,” Kalaga said. “The clay-based electrolyte gets less viscous but still retains its consistency at least to 150˚ C. The next step is to push the limits further.”
Source: Rice University via Green Car Congress
Top graphic by Kaushik Malaga