Lithium metal is considered an ideal anode material due to its exceptional charge and storage capability, but it has a tragic flaw: root-like growths called dendrites, which can lead to short-circuiting and spontaneous combustion.
Now, researchers at the University of Maryland (UMD) have announced a breakthrough in their quest to solve the dendrite issue. In “Highly Fluorinated Interphases Enable High-Voltage Li-Metal Batteries,” published in Chem, Professor Chunsheng Wang and colleagues explain how they created a battery chemistry that successfully suppressed dendrite formation in Li-metal batteries by increasing the LiFSI (lithium bis[fluorosulfonyl]imide) salt concentration in the electrolyte.
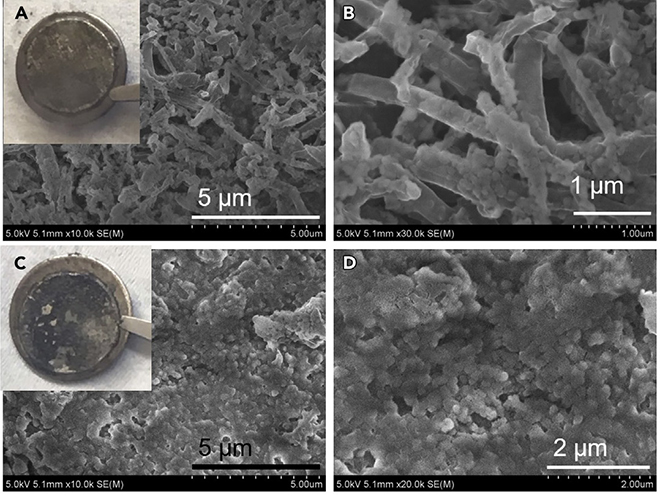
“Dendrite formation in the Li battery could penetrate the separator and cause serious safety issues,” said lead author Xiulin Fan. “This FSI anion in the concentrated electrolyte will react with the lithium metal anode to generate a LiF-rich SEI layer, which can suppress dendrite formation and greatly improve the coulombic efficiency for the Li metal anode.”
“The capacity of [a graphite anode] is less than 372 mAh/g,” Dr. Fan continued. “The Li-metal anode can deliver a capacity as high as 3,860 mAh/g. So, if we replace the graphite with a Li-metal anode, the capacity and the energy density for the Li-battery could be doubled.”
Source: University of Maryland