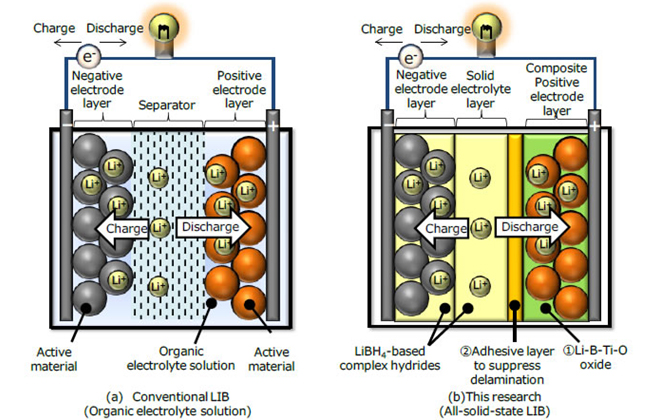
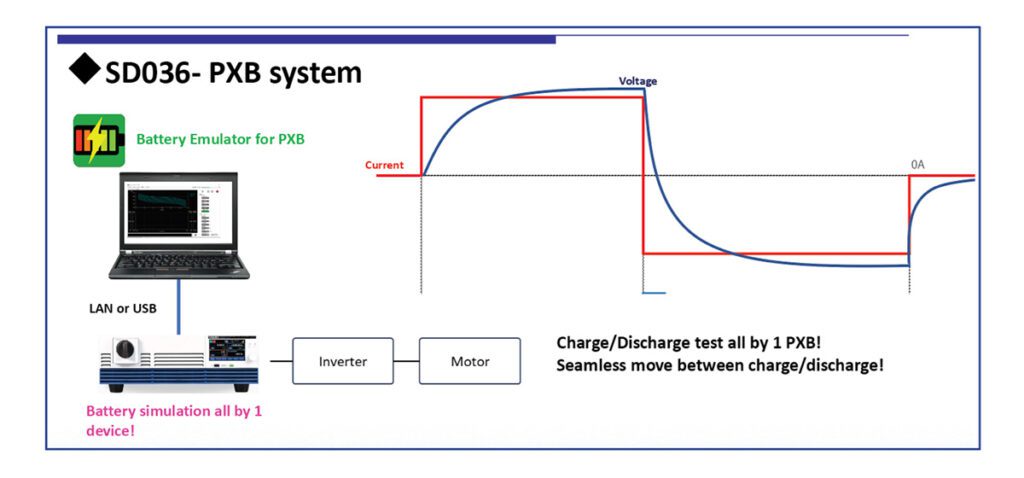
A research team sponsored by Hitachi and Tohoku University’s Advanced Institute for Material Research (AIMR) has demonstrated technology that reduces the internal resistance of all-solid-state Li-ion batteries through the use of LiBH4-based complex hydrides as solid electrolytes.
A conventional Li-ion battery uses a volatile organic electrolyte solution with a maximum operating temperature around 60˚ C. Automotive batteries need to operate in higher ambient temperatures, which is why they generally require some sort of cooling system. While non-volatile solid electrolytes have been developed for use in high-temperature environments, their lithium ion conductivity is lower than that of an organic electrolyte. Thus, the key to making an all-solid-state Li-ion battery commercially viable is reducing its internal resistance.
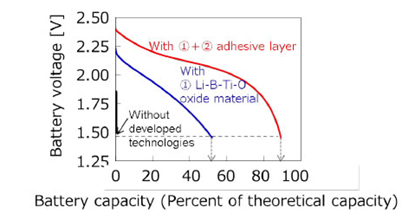
Professor Shin-ich Orimo and his colleagues have been conducting research on LiBH4-based complex hydrides as novel solid electrolytes, and have confirmed fast lithium-ion conductivity from room temperature to 150 ℃. They were able to reduce the cell’s internal resistance, improving charge-discharge performance. The team’s batteries have a capacity of 2 mAh, density of 30 Wh/L, and a discharge capacity of 90% of theoretical value.
This technology could allow the thermally durable Li-ion battery to be used in a wider variety of applications. Because it does not require the cooling system common in conventional batteries, Hitachi expects it to lead to the development of more compact battery systems, reducing overall costs.
Source: Hitachi via Green Car Congress