Magnesium batteries have long been considered a potentially safer and less expensive alternative to lithium-ion batteries, but previous versions have been severely limited in the amount of power they delivered.
Researchers from the University of Houston and the Toyota Research Institute of North America (TRINA) reported in Nature Energy that they have developed a new cathode and electrolyte—previously the limiting factors for a high-energy magnesium battery—to demonstrate a magnesium battery capable of operating at room temperature and delivering a power density comparable to that offered by lithium-ion batteries.
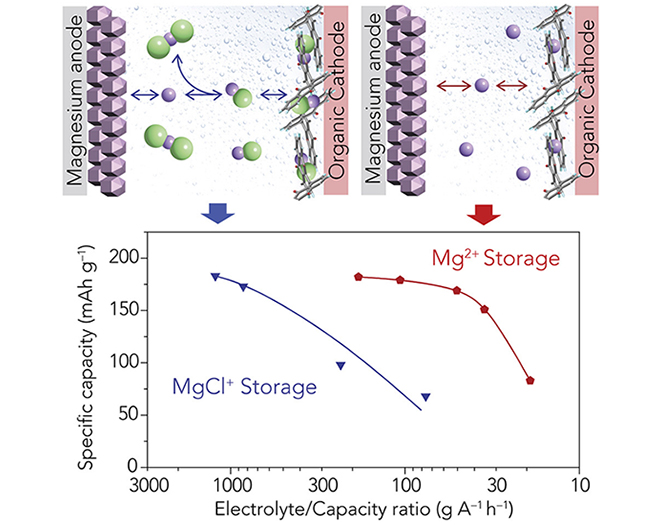
Magnesium ions can hold twice the charge compared to lithium, and have a similar ionic radius. As a result, magnesium dissociation from electrolytes and its diffusion in the electrode, two essential processes that take place in classical intercalation cathodes, are sluggish at room temperature, leading to low power performance.
One of the paper’s co-authors, Professor Yan Yao, said the results came from combining both an organic quinone cathode and a newly-tailored boron cluster-based electrolyte solution.

“We demonstrated a heterogeneous enolization redox chemistry to create a cathode which is not hampered by the ionic dissociation and solid-state diffusion challenges that have prevented magnesium batteries from operating efficiently at room temperature,” Yao said. “This new class of redox chemistry bypasses the need for solid-state intercalation while solely storing magnesium, instead of its complex forms, creating a new paradigm in magnesium battery electrode design.”
TRINA researchers have made advancements in magnesium batteries, including the development of highly recognized, efficient electrolytes based on boron cluster anions. However, these electrolytes had limitations in supporting high battery cycling rates.
“We had hints that electrolytes based on these weakly coordinating anions in principle could have the potential to support very high cycling rates, so we worked on tweaking their properties,” said Rana Mohtadi, a Principal Scientist at TRINA and another of the paper’s co-authors. “We tackled this by turning our attention to the solvent in order to reduce its binding to the magnesium ions and improve the bulk transport kinetics. We were fascinated that the magnesium plated from the modified electrolyte remained smooth even under ultrahigh cycling rates. We believe this unveils a new facet in magnesium battery electrochemistry.”
The work is in part a continuation of earlier efforts described in 2018 in Joule that involved many of the same researchers.
“The new battery is nearly two orders of magnitude higher than the power density achieved by previous magnesium batteries,” said post-doctoral researcher Hui Dong, who also contributed to the paper. “The battery was able to continue operating for over 200 cycles with around 82% capacity retention, showing high stability. We can further improve cycling stability by tailoring the properties of the membrane with enhanced intermediate trapping capability.”
Source: University of Houston