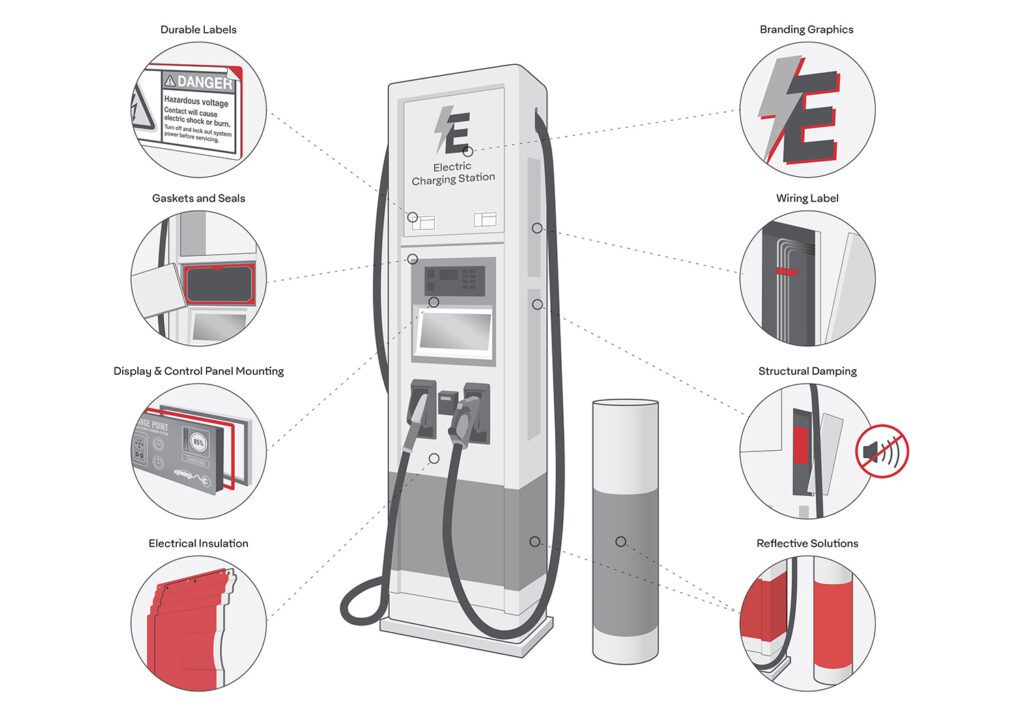
Researchers from Carnegie Mellon University have developed a semi-liquid lithium metal-based anode that could lead to higher capacity and better safety than typical lithium metal-based batteries that use lithium foil as anodes. The research team published their findings in the June 2019 issue of Joule.
“Incorporating a mewtallic lithium anode into lithium-ion batteries has the theoretical potential to create a battery with much more capacity than a battery with a graphite anode,” said Krzysztof Matyjaszewski, Professor of Natural Sciences at Carnegie Mellon. “But, the most important thing we need to do is make sure that the battery we create is safe.”
After a lithium-based battery has been charged and discharged repeatedly, strands of lithium called dendrites can grow on the surface of the electrode. The dendrites can pierce through the membrane that separates the two electrodes. This allows contact between the anode and cathode, which can cause the battery to short circuit and, in the worst case, catch fire.
One proposed solution to the volatile liquid electrolytes used in current batteries is to replace them with solid ceramic electrolytes. These electrolytes are highly conductive, non-combustible and strong enough to resist dendrites. However, researchers have found that the contact between the ceramic electrolyte and a solid lithium anode is insufficient for storing and supplying the amount of power needed for most electronics.
Carnegie Mellon doctoral students Sipei Li and Han Wang were able to surmount this shortcoming by creating a new class of material that can be used as a semi-liquid metal anode.
Working with Matyjaszewski and engineering professor Jay Whitacre, Li and Wang created a dual-conductive polymer/carbon composite matrix that has lithium microparticles evenly distributed throughout. The matrix remains flowable at room temperatures, which allows it to create a sufficient level of contact with the solid electrolyte. By combining the semi-liquid metal anode with a garnet-based solid ceramic electrolyte, they were able to cycle the cell at 10 times higher current density than cells with a solid electrolyte and a traditional lithium foil anode. This cell also had a much longer cycle-life than traditional cells.
The researchers believe that their method could have far reaching impacts. It could be used to create high capacity batteries for EVs and flexible batteries for wearable devices. They also believe that their methods could be extended beyond lithium to other rechargeable battery systems, including sodium metal batteries and potassium metal batteries.
“This new processing route leads to a lithium metal-based battery anode that is flowable and has very appealing safety and performance compared to ordinary lithium metal,” said Whitacre. “Implementing new material like this could lead to step change in lithium-based rechargeable batteries, and we are working hard to see how this works in a range of battery architectures.”
Source: Carnegie Mellon University