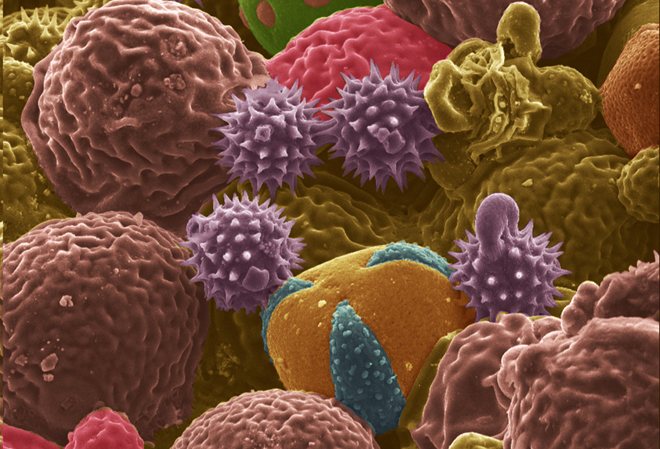
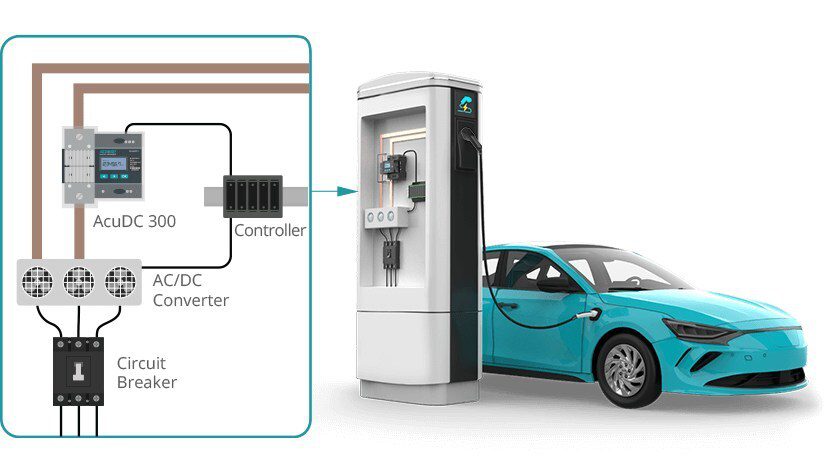
Much research in the battery world focuses on finding a replacement for graphite as an anode material. One candidate is hard carbon, which can be formed in various morphologies to deliver the desired properties. But in fact, there are already millions of tiny factories around the world, cranking out as many carbon particles as could be desired.
A team at Purdue University has found a way to use plant pollens as the basis for carbon microstructures. Plants produce pollen in a vast variety of shapes and sizes, some of which fall in the range of commercial carbon anode particle sizes.
In “From Allergens to Battery Anodes: Nature-Inspired, Pollen Derived Carbon Architectures for Room-and Elevated-Temperature Li-ion Storage,” published in Nature’s Scientific Reports, Jialiang Tang and Vilas Pol explain how they air-activated the carbonaceous particles, forming pores to increase their energy-storage capacity, and then evaluated them as lithium-ion battery anodes at room (25° C) and elevated (50° C) temperatures. They found that an activated cattail pollen electrode delivered high specific lithium storage reversible capacities (590 mAh/g at 50° C and 382 mAh/g at 25° C) and also exhibited excellent high rate capabilities.
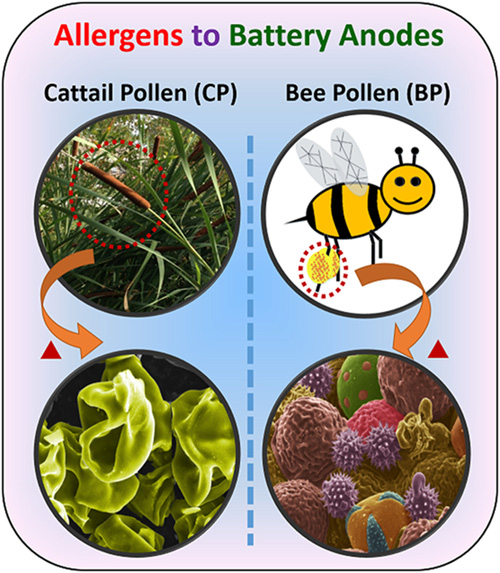
“Herein, we report the conversion of allergenic pollen grains into carbon microstructures through a facile, one-step, solid-state thermochemical decomposition in an inert atmosphere at elevated temperatures,” write Tang and Pol. “For this study, carbon derived from both cattail pollens and bee pollens were evaluated for their potential application in LIB anodes. Cattail pollen was chosen to represent pollens with selective morphology but with limited commercial supply, while bee pollens represent pollens with unselective/diverse morphologies but are commercially available in large quantities.”
The next step is to study the pollen-based anodes in a full-cell battery with a commercial cathode.
Source: Purdue University via Green Car Congress
Images: Purdue University/Jialiang Tang