Chemists at Canada’s University of Waterloo have described a key mediation pathway that explains why sodium-oxygen batteries are more energy-efficient compared with their lithium-oxygen counterparts.
“Our new understanding brings together a lot of different, disconnected bits of a puzzle that have allowed us to assemble the full picture,” says Professor Linda Nazar. “These findings will change the way we think about non-aqueous metal-oxygen batteries.”
In a paper published in the journal Nature Chemistry, Nazar and her colleagues describe their discovery of the so-called proton phase transfer catalyst. By isolating its role in the battery’s discharge and recharge reactions, they were able to boost the battery’s capacity, and achieve a near-perfect recharge of the cell.
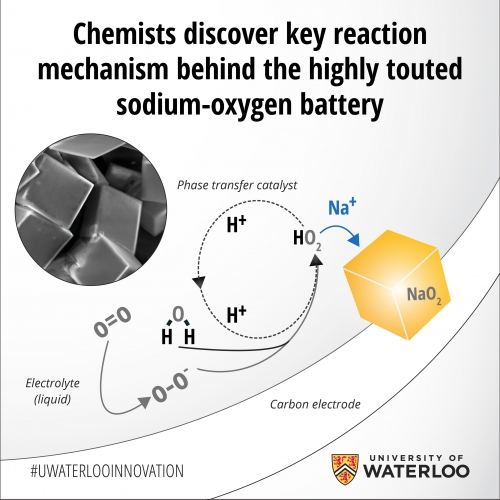
Unlike the traditional solid-state battery design, a metal-oxygen battery uses a gas cathode that combines oxygen with a metal such as sodium or lithium to form a metal oxide, storing electrons in the process. Applying an electric current reverses the reaction and returns the metal to its original form.
In the case of the sodium-oxygen cell, the proton phase catalyst transfers the newly formed sodium superoxide (NaO2) entities to solution, where they nucleate into well-defined nanocrystals to grow the discharge product as micron-sized cubes. When the battery is recharged, these NaO2 cubes readily dissociate, with the reverse reaction facilitated once again by the proton phase catalyst.
The proton phase catalyst could theoretically work with lithium-oxygen, but the lithium superoxide (LiO2) entities are too unstable and convert to lithium peroxide (Li2O2). Once Li2O2 forms, the catalyst cannot facilitate the reverse reaction. In order to make progress on lithium-oxygen systems, researchers need to find an additional redox mediator.
“We are investigating redox mediators as well as exploring new opportunities for sodium-oxygen batteries that this research has inspired,” said Nazar.” Lithium-oxygen and sodium-oxygen batteries have a very promising future, but their development must take into account the role of how high capacity – and reversibility – can be scientifically achieved.”
Source: University of Waterloo via Green Car Congress