The lithium-ion battery market is demanding higher energy density, extended lifetime, and improved power performance. Arkema’s Battery Solutions address many of these demands. Advances in electrode materials are addressing energy density, while new electrolyte systems can significantly improve lifetime and power performance. LiFSI, in particular, is a good candidate to replace LiPF6, which is widely used as the lithium salt in the electrolyte.
Today, LiFSI is mostly used as an additive in the electrolyte while LiPF6 remains the main lithium salt. A small amount of LiFSI can improve low temperature performance, lifetime, and storage stability due to its interaction in the solid electrolyte interface.
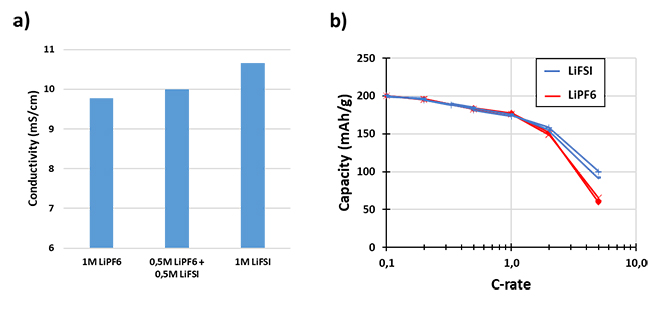
Furthermore, LiFSI can achieve even better performance with a larger replacement of LiPF6. Replacement of LiPF6 with LiFSI drives higher ionic conductivity (Figure 1a) and a higher transference number for the electrolyte. This enables LiFSI-based electrolytes to have improved rate performance in NMC811/graphite cells compared to LiPF6-based electrolyte (Figure 1b).
Figure 1: Ionic Conductivity and Rate Performance of LiFSI Electrolyte Solutions
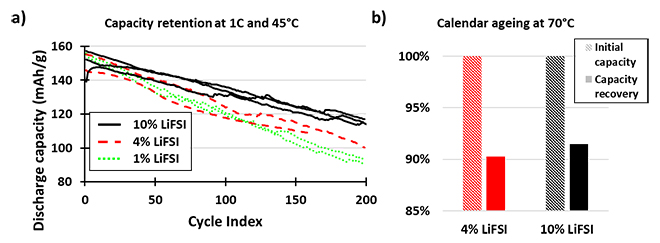
Increasing the LiFSI/LiPF6 ratio enhances the lifetime at 45°C as well as calendar aging due to the improved thermal and chemical stability of LiFSI, which tends to generate less HF than LiPF6. There is improved capacity retention of NMC532/graphite cells after 200 cycles with higher concentrations of LiFSI in the electrolyte; 1wt%, 4wt%, and 10wt% by weight of the electrolyte, the remaining salt being LiPF6 (Figure 2a). The capacity recovery of NMC/graphite cells stored at 100% SOC at 70°C for two weeks is also improved with 10wt% rather than 4wt% of LiFSI in the electrolyte (Figure 2b).
Figure 2: Capacity Retention and Calendar Aging for LiFSI Electrolytes
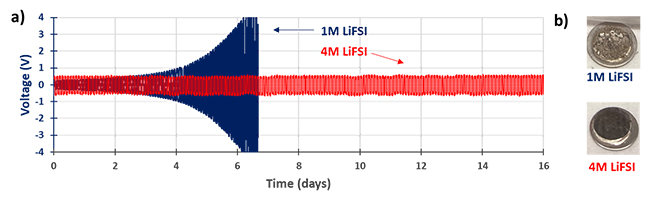
Another advantage of LiFSI is its high solubility, which facilitates highly concentrated electrolytes. Such electrolytes are gaining interest because the salt/solvent coordination structure can alleviate or even suppress the electrolyte flammability, decomposition at high voltage, and dendrite growth on lithium metal anodes. In a dendrite test at 0.5 mA/cm² with 1M LiFSI in DME, dendrite growth is observed after a few days of continuous lithium stripping and plating, as revealed through the increase of cell polarization (Figure 3a). On the other hand, in the highly concentrated electrolyte (4M LiFSI in DME), dendrite formation is suppressed thanks to a more stable and compact lithium-rich SEI formed on the lithium metal (Figure 3b). After several days of cycling, the surface of lithium metal in the low-concentration electrolyte (1M) is thick and uneven while the SEI of the lithium metal in the highly concentrated electrolyte (4M) is smoother, with no dead lithium.
Figure 3: LiFSI Performance in Lithium Metal Anode Cells
Importantly, the purity of LiFSI salt becomes critical when LiFSI is used at a higher concentration rather than as a mere additive. In particular, certain ions like fluorine, sulfate or chlorine resulting from the synthesis can adversely affect the cell performance ― even in small amounts such as a few ppm ― through different mechanisms: increasing the side reactions of the electrolyte at high voltage, interacting in the SEI and prompting lithium plating, or even inducing the corrosion of aluminum current collector.
Arkema is producing ultra-high purity LiFSI salt under the FORANEXT® trademark. This salt can effectively passivate aluminum even in the absence of LiPF6 (Figure 4); the residual current measured in a Li/Al coin cell at 4.4V is very low and stable with 1M LiFSI in EC/EMC (3:7, vol.).
Figure 4: Chronoamperometry at 4.4V

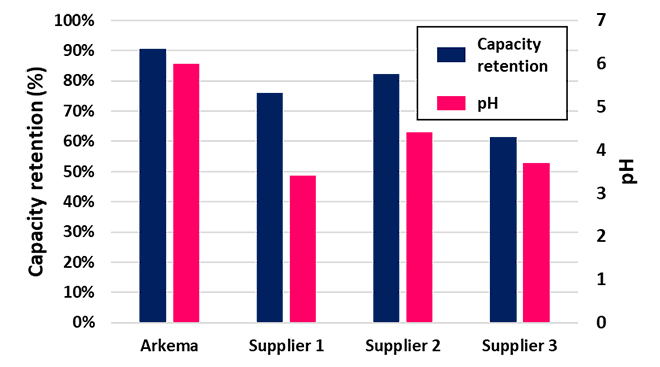
In order to benchmark FORANEXT® LiFSI against other LiFSI available on the market, the pH value in aqueous solution of LiFSI was measured. The presence of acidic species may enable the formation of HF during cycling or storage, decreasing the pH and indicating lower purity over time. Comparing the pH values and capacity retention of aqueous solutions made with different LiFSI sources after cycling, ultra-high purity FORANEXT® LiFSI showed the best performance (Figure 5). Since capacity retention depends on the nature of the specific impurities, there is not always a direct correlation between pH and capacity retention. However, by reducing all impurities, FORANEXT® LiFSI exhibits the best capacity retention after 200 cycles, which highlights the need for ultra-high purity LiFSI when used for large replacement of LiPF6.
Figure 5: pH and Capacity Retention of Different LiFSI Sources
In conclusion, partial substitution of LiPF6 with LiFSI in the electrolyte enables improvement of the rate performance and capacity retention of lithium-ion cells. LiFSI is also a promising alternative to LiPF6 for the development of new lithium-ion battery technology with higher energy density such as lithium metal batteries. For applications that use LiFSI at higher concentrations than additive quantities, ultra-high purity of LiFSI, like FORANEXT® LiFSI, is required in order to achieve the best performance.
Learn more about all of Arkema’s Battery Solutions for inside the cell, cell to module assembly, battery pack sealing and enclosure, and battery management systems. For more information, follow Arkema Battery Solutions on LinkedIn for updates on the latest innovations.






















































































![New York City may replace Central Park’s horse-drawn cabs with electric carriages [Updated]](https://chargedevs.com/wp-content/uploads/2025/09/AdobeStock_273233016.jpg)




