We have all heard the comforting bromide about our imperfections being our greatest strengths. Rice University researchers have recently proven this platitude in the context of battery charging cycles with the discovery that defects purposely inserted into the crystalline lattice of a lithium iron phosphate cathode actually cause the battery to work more, not less, efficiently. The team published its findings in the journal npj Computational Materials.
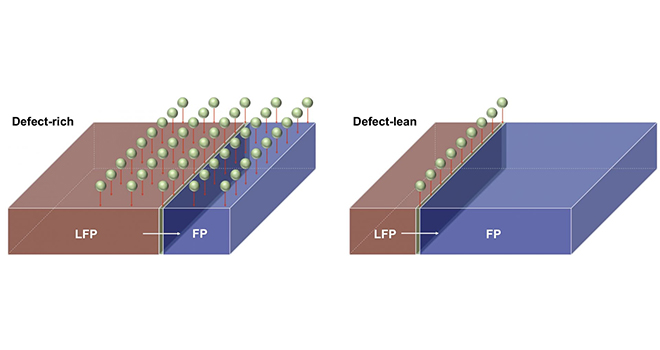
Charging a cathode essentially means inciting phase transition from iron phosphate to lithium iron phosphate and going from a lithium-poor to a lithium-rich surface. Defects placed in the crystalline lattice of the cathode appear to make the charging cycle improve by up to two orders of magnitude. What is referred to as a “defect” simply involves placing an atom where it should not technically be on the lattice. This means iron is seated where lithium would be expected, and vice versa. Generally, these kind of rearranged atomic table settings known as “antisites” spell slower lithium movement within the crystal lattice and thus decreased battery performance. However, the Rice researchers have demonstrated that lithium iron phosphate makes highly useful detours out of these defects, resulting in the lithium ions connecting with the reaction boundary over a much broader surface area. This in turn directly enhances the efficiency of the battery cycle by allowing for more uniform lithium exposure across the cathode.
With support from the DOE, researcher Ming Tang and colleagues have shown that spreading defects across a cathode surface not only greatly aids in lithium insertion, but also in reducing damage caused to a cathode when high voltage is applied in order to get a fast charge. “An interesting prediction of the model is that this optimal defect configuration depends on the shape of the particles,” says Tang. “We saw that facets of a certain orientation could make the detours more effective in transporting lithium ions. Therefore, you will want to have more of these facets exposed on the cathode surface.” Tang envisions using this same brand of defect optimization for the improvement of all sorts of phase-changing batteries in the future, and cites steel and ceramics as structural materials wherein similar experiments have shown that defects can generate beneficial material strengths.
Sources: Rice University