Lithium-air, or lithium-oxygen, has been touted as the ultimate battery technology, because its theoretical energy density is ten times that of current lithium-ion solutions. However, scientists have struggled to overcome major challenges with the technology. The batteries waste a lot of energy as heat, degrade relatively quickly, and often require extra components to pump oxygen gas in an open-cell configuration that is very different from conventional sealed batteries.
In “Anion-redox nanolithia cathodes for Li-ion batteries,” reported in the journal Nature Energy, researchers demonstrated a new variation of the battery chemistry, which could be used in a conventional, fully sealed battery while overcoming the major drawbacks. The paper’s authors are Ju Li, the Battelle Energy Alliance Professor of Nuclear Science and Engineering at MIT; postdoc Zhi Zhu; and five others at MIT, Argonne National Laboratory, and Peking University in China.
Conventional lithium-air batteries draw in oxygen from the outside air to drive a chemical reaction with the battery’s lithium during the discharging cycle. The oxygen is then released again to the atmosphere during the reverse reaction in the charging cycle. In this new approach, the same kind of electrochemical reactions take place without letting the oxygen revert to a gaseous form. Instead, the oxygen stays inside a solid and transforms directly between three redox states, while bound in the form of three different solid chemical compounds which are mixed together in the form of a glass.
This reduces the voltage loss by a factor of five, from 1.2 volts in other lithium-air technologies to 0.24 volts, so only 8 percent of the electrical energy is turned to heat. “This means faster charging for cars, as heat removal from the battery pack is less of a safety concern, as well as energy efficiency benefits,” Li says.
This approach helps overcome another issue with lithium-air batteries: As the chemical reaction involved in charging and discharging converts oxygen between gaseous and solid forms, the material goes through huge volume changes that can disrupt electrical conduction paths in the structure, severely limiting its lifetime.
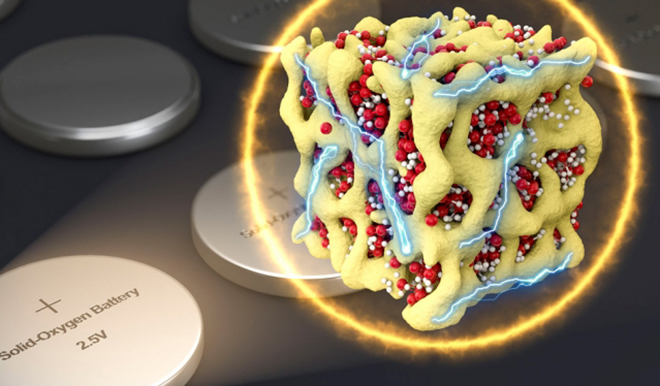
The new formulation creates minuscule particles, at the nanometer scale, which contain both the lithium and the oxygen in the form of a glass, confined tightly within a matrix of cobalt oxide. The researchers refer to these particles as nanolithia.
The nanolithia particles would normally be very unstable, so the researchers embedded them within the cobalt oxide matrix, a sponge-like material with pores just a few nanometers across. The matrix stabilizes the particles and also acts as a catalyst for their transformations.
The new battery is also inherently protected from overcharging, the team says, because the chemical reaction in this case is naturally self-limiting — when overcharged, the reaction shifts to a different form that prevents further activity.
In cycling tests, a lab version of the new battery was put through 120 charging-discharging cycles, and showed less than a 2 percent loss of capacity, indicating that such batteries could have a long useful lifetime. And because such batteries could be installed and operated just like conventional solid lithium-ion batteries, without any of the auxiliary components needed for a lithium-air battery, they could be easily adapted to existing installations or conventional battery pack designs for cars, electronics, or even grid-scale power storage.
Because these “solid oxygen” cathodes are much lighter than conventional lithium-ion battery cathodes, the new design could store as much as double the amount of energy for a given cathode weight, the team says. And with further refinement of the design the new batteries could ultimately double that capacity again.
Overall, the new battery system is “very scalable, cheap, and much safer” than lithium-air batteries, said Li.
The team expects to move from this lab-scale proof of concept to a practical prototype within a year.
Source: MIT, Nature Energy