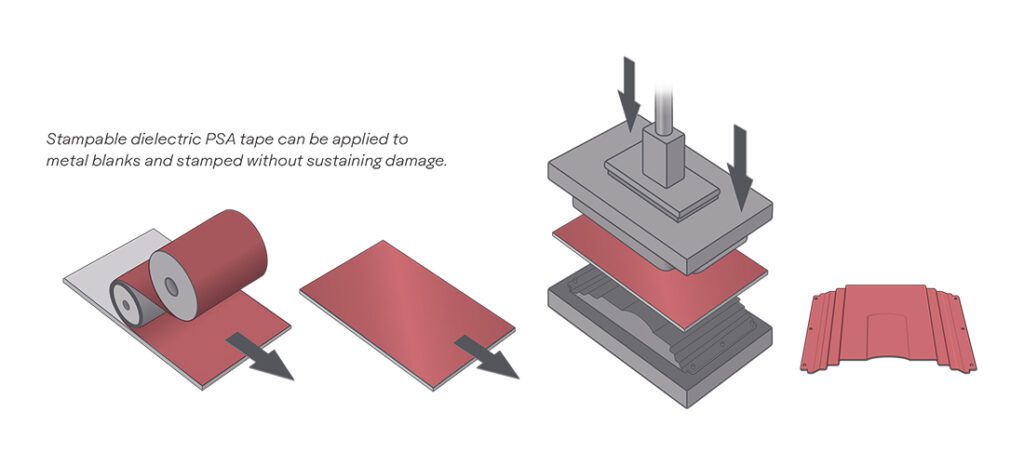
MIT Associate Professor of Metallurgy Antoine Allanore’s lab has received a nearly $2-million dollar grant to further develop copper collection methods from molten sulfur-based minerals using electricity. The resultant high-purity copper could be used in the production of high-quality wire used in growing markets like renewable energy and EVs.
Current pyrometallurgy methods are tedious: sulfide minerals must be crushed, copper-rich sections must be floated away, the copper is refined in a smelter and then finally subjected to electrolysis for purification. These methods produce toxic sulfur dioxide and copper with large amounts of sulfur and oxygen. Allanore’s lab previously developed a one-step electrolysis technique for the separation of copper from molten sulfide mineral ore that produces purer copper. The process also has the added benefit of creating elemental sulfur as a byproduct instead of sulfur dioxide.
The lab’s studies also resulted in the production of copper, molybdenum, and rhenium from sulfur-rich minerals using a technique similar to the Hall-Héroult process of aluminum production with much higher operating temperatures. This grant will allow Allanore’s group to create a reactor capable of producing ten times the copper per hour while running longer and providing more data about the other valuable metals extracted in the process. The goal is to develop a pilot plant in three years. “We are aiming to be ready to provide the design criteria, the material and operating conditions of a one metric tonne [about 2,204 pounds, or 1.1 U.S. tons] per day demonstration reactor,” says Allanore.
“The revolution that we are proposing is that only one reactor would do everything. It would make the liquid copper product and allow us to recover elemental sulfur, and allows us to recover selenium,” says Allanore. “It may be possible to cut the energy needed for making copper by 20 percent.”
“If developed and deployed, it has the potential to decrease energy demand, operate entirely on renewable energy, and reduce sulfur dioxide emissions,” says ICA Technology Director Hal Stillman. “In addition, it can separate unwanted impurities and recover valuable by-products from the concentrate. Right now, the technical evidence that is creating excitement is a small-scale proof-of-principle demonstration.”
Source: MIT