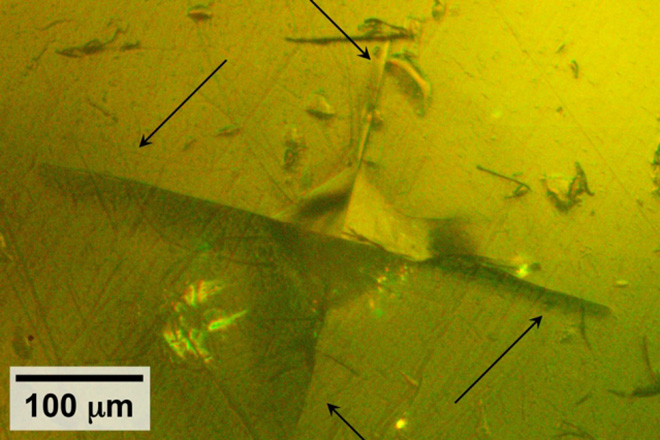
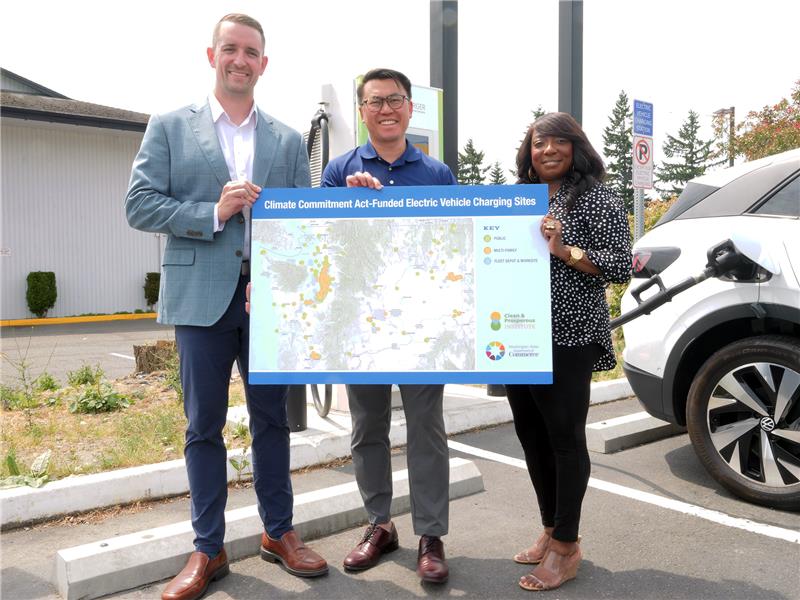
IMAGE: Using specialized equipment, a team from MIT did tests in which they used a pyramidal-tipped probe to indent the surface of a piece of the sulfide-based material. Surrounding the resulting indentation (seen at center), cracks were seen forming in the material (indicated by arrows), revealing details of its mechanical properties.
Battery scientists are the world are exploring the possibility of all-solid-state batteries, in which the liquid electrolyte is replaced by a solid electrolyte enhancing the batteries’ energy density and safety.
A team at MIT has probed the mechanical properties of a sulfide-based solid electrolyte material, to determine its mechanical performance when incorporated into batteries. The new findings from the group – led by Frank McGrogan, Tushar Swamy, Krystyn Van Vliet, and Ming Chiang – were published in the journal Advanced Energy Materials.
In the lithium-ion batteries that dominate the market today, lithium ions pass through a liquid electrolyte to get from one electrode to the other while the battery is being charged, and then flow through in the opposite direction as it is being used. These batteries are very efficient, but “the liquid electrolytes tend to be chemically unstable, and can even be flammable,” said Van Vliet. “So if the electrolyte was solid, it could be safer, as well as smaller and lighter.” Solid electrolytes may also virtually eliminate the risk of tiny, fingerlike metallic projections called dendrites that can grow through the electrolyte layer and lead to short-circuits.
The big question is what kinds of mechanical stresses might occur within the electrolyte material as the electrodes charge and discharge repeatedly. This cycling causes the electrodes to swell and contract as the lithium ions pass in and out of their crystal structure. In a stiff electrolyte, those dimensional changes can lead to high stresses and cracks.
Lithium-conducting sulfide is considered a promising candidate for electrolytes in all-solid-state batteries. However, the sulfide’s extreme sensitivity to normal lab air has posed a challenge to measuring mechanical properties including its fracture toughness. To circumvent this problem, members of the research team conducted the mechanical testing in a bath of mineral oil, protecting the sample from any chemical interactions with air or moisture, and were able to obtain detailed measurements of the mechanical properties of the sulfide.
The researchers found that the material has a combination of properties somewhat similar to silly putty or salt water taffy: When subjected to stress, it can deform easily, but at sufficiently high stress it can crack like a brittle piece of glass. The team concluded that the material is more brittle than would be ideal for battery use, but as long as its properties are known and systems designed accordingly, it could still have potential for such uses, McGrogan says. “You have to design around that knowledge.”
Source: MIT