Researchers from the Korea Advanced Institute of Science and Technology (KAIST) have discovered a means to extend the lifetime of lithium-air (Li-air) batteries, a technology that offers the promise of higher energy densities than those achievable with conventional lithium-ion batteries. They report their findings in the February issue of the journal ChemSusChem.
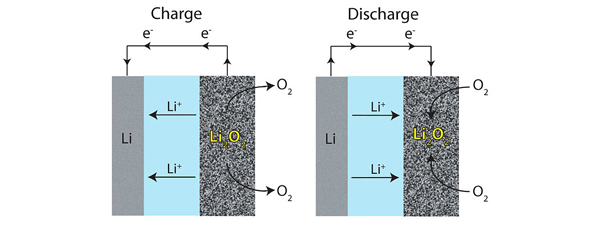
Li-air batteries are hindered by problems surrounding the high-energy free radicals generated at their cathodes. These radicals, which are oxygen molecules with an extra electron, have a tendency to interact with lithium electrodes, leading to the production of chemical species that are difficult to break down. Reversing the synthesis of these chemical species is deemed to be the greatest challenge for Li-air technology by the researchers at KAIST and their unique solution was to combine two literal opposites, the solvent propylene carbonate (PC) and an ionic liquid (IL).
Engineering Details:
PC has the desirable trait of low volatility but it reacts with radical oxygens to create irreversible chemical species and radical oxygens act destructively on it, leading to a scarcity of PC molecules. This scarcity depletes the system of usable sites to carry out ion transfer, robbing the battery of its capacity to generate power. ILs, on the other hand, are attractive due to their very low flammability, low vapor pressure and stability upon heating. By themselves, however, they have low oxygen solubilities and high viscosities which limit performance by slowing down the motion of ions.
By mixing an IL with a PC, the researchers found that these solvents improve experimental battery lifetime considerably, each component making up for the limitations of the other. The IL, PYR13-TFSI, did not suffer from the production of irreversible by-products and the PC has a sufficiently low viscosity – therefore high enough ionic conductivity and oxygen solubility – so that the blended cells have more practical voltage than pure IL. Meanwhile, the PYR13+ ion is effective at neutralizing the radical oxygen molecules that would otherwise consume the PC. In tests involving 70 charge-discharge cycles, cells containing 50% IL kept 94.6% of their capacity and had a higher initial capacity than cells starting with either more PC or more IL. That level of stability is unprecedented for carbonate electrolytes.
As for the future of this innovation, associate professor and project supervisor Jang Wook Choi indicates that the KAIST team’s research is still nascent: “We need to develop other cell components that can be integrated better with the current electrolyte…perhaps under collaboration with industry counterparts nearby.”
On the subject of commercialization, Choi is pragmatic. “[It] may not be in the near future. However, once Li-air cells become upgraded further by developing and optimizing all of the relevant cell components, the current approach could be a viable option or at least a useful stepping stone for bringing the Li-air battery technology to the commercial level.”
In terms of safety, Dr. Choi told Charged he believes that the presence of an IL will improve this parameter. “However, other properties such as lifetime and rate performance, rather than safety, should be improved substantially for this type of cell to be more feasible.”
Source: KAIST, ChemSusChem
Image: Na9234 (wikipedia)