With a fresh round of funding, Wildcat Discovery Technologies is busy expanding its facilities, building up its battery team and doubling down on promising solid-state research projects. We asked the energy storage experts to help clear up the hype around solid-state batteries.
Whenever Charged needs to better understand the complexities of the latest and greatest battery tech—or the nuances of the battery materials industry—our first call is to Wildcat Discovery Technologies. This group of energy storage scientists has a unique industry perspective, because they use their proprietary high-throughput research platform to help other companies improve their batteries by rapidly exploring new materials. Wildcat is at the forefront of the race to find better battery materials, and its combinatorial chemistry techniques can build and test prototype batteries up to 10 times faster than a standard lab—a process that’s ideally suited for optimizing materials with seemingly endless formulation possibilities.
The result is that Wildcat not only understands the deep technical details of all the top battery chemistries, but also has a great perspective on the commercialization timelines for what’s considered next-generation technology.
With the explosion of interest in battery tech in the past decade, the company has been busy. Wildcat recently closed a new investment round of over $20 million, upgraded its lab in a new facility, hired top battery experts, began discussions to license its R&D platform, and expanded its internal projects into key areas of solid-state battery tech.
We come across a lot of press release hyperbole and confusing terms/concepts surrounding solid-state battery technology. So Charged recently chatted with Wildcat to discuss the latest in its battery research, including the race for solid-state batteries and the quest for the top prize: a solid electrolyte cell with a lithium metal anode.
The state of solid-state
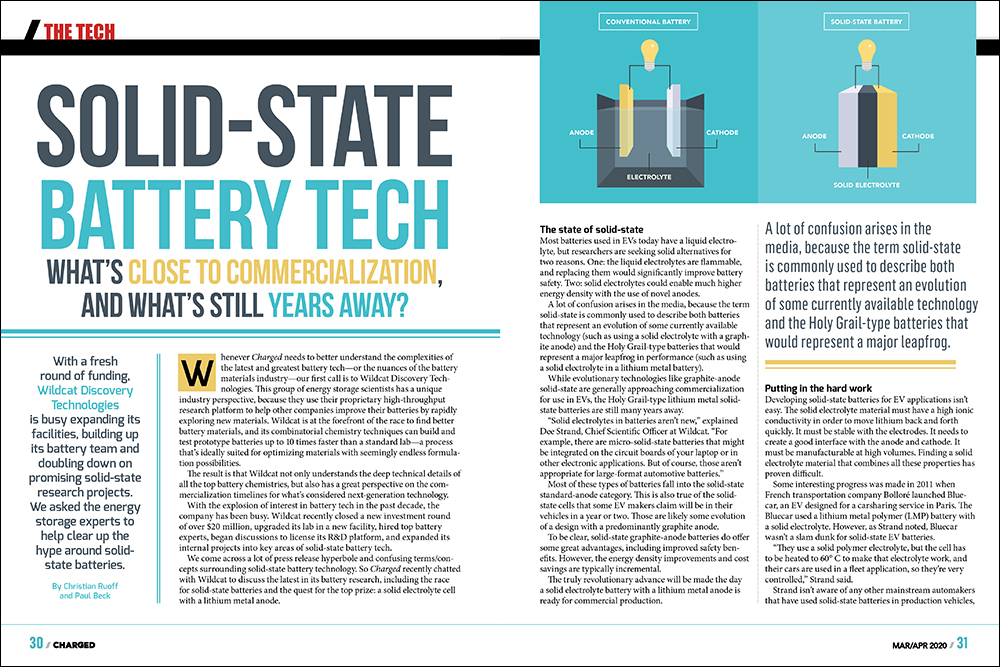
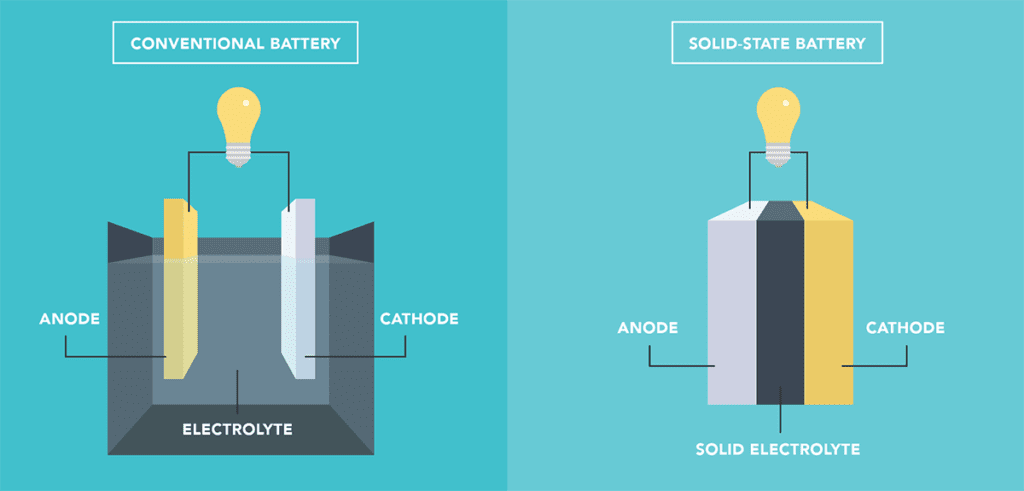
Most batteries used in EVs today have a liquid electrolyte, but researchers are seeking solid alternatives for two reasons. One: the liquid electrolytes are flammable, and replacing them would significantly improve battery safety. Two: solid electrolytes could enable much higher energy density with the use of novel anodes.
A lot of confusion arises in the media, because the term solid-state is commonly used to describe both batteries that represent an evolution of some currently available technology (such as using a solid electrolyte with a graphite anode) and the Holy Grail-type batteries that would represent a major leapfrog in performance (such as using a solid electrolyte in a lithium metal battery).
While evolutionary technologies like graphite-anode solid-state are generally approaching commercialization for use in EVs, the Holy Grail-type lithium metal solid-state batteries are still many years away.
“Solid electrolytes in batteries aren’t new,” explained Dee Strand, Chief Scientific Officer at Wildcat. “For example, there are micro-solid-state batteries that might be integrated on the circuit boards of your laptop or in other electronic applications. But of course, those aren’t appropriate for large-format automotive batteries.”
Most of these types of batteries fall into the solid-state standard-anode category. This is also true of the solid-state cells that some EV makers claim will be in their vehicles in a year or two. Those are likely some evolution of a design with a predominantly graphite anode.
To be clear, solid-state graphite-anode batteries do offer some great advantages, including improved safety benefits. However, the energy density improvements and cost savings are typically incremental.
The truly revolutionary advance will be made the day a solid electrolyte battery with a lithium metal anode is ready for commercial production.
Putting in the hard work
Developing solid-state batteries for EV applications isn’t easy. The solid electrolyte material must have a high ionic conductivity in order to move lithium back and forth quickly. It must be stable with the electrodes. It needs to create a good interface with the anode and cathode. It must be manufacturable at high volumes. Finding a solid electrolyte material that combines all these properties has proven difficult.
Some interesting progress was made in 2011 when French transportation company Bolloré launched Bluecar, an EV designed for a carsharing service in Paris. The Bluecar used a lithium metal polymer (LMP) battery with a solid electrolyte. However, as Strand noted, Bluecar wasn’t a slam dunk for solid-state EV batteries.
“They use a solid polymer electrolyte, but the cell has to be heated to 60° C to make that electrolyte work, and their cars are used in a fleet application, so they’re very controlled,” Strand said.
Strand isn’t aware of any other mainstream automakers that have used solid-state batteries in production vehicles, but many are keenly exploring the technology. Toyota, for instance, has conducted research into solid-state batteries using sulfide-based electrolytes. And this past October, Toyota Chief Technology Officer Shigeki Terashi told Autocar, “We will produce a car with solid-state batteries and unveil it to you in 2020, but mass production with solid-state batteries will be a little later.”
“The advantage of sulfide is that it has really high ionic conductivity,” Strand said. “The downside is that there are stability issues with the anode or cathode, so you have to have some protective coating. It’s also challenging from a manufacturing standpoint, because it can generate some toxic gases if it’s exposed to moisture, so there are some safety concerns with sulfides that need to be addressed.”
Other candidates for solid electrolyte materials include polymers and ceramics. However, none of those solutions have entered EV-scale production either, and in the near term, they will likely include some form of a graphite anode without the step-change improvements promised by lithium metal.
“The value proposition of a solid-state battery that uses a conventional graphite anode cell is more limited,” Jon Jacobs, VP of Business Development at Wildcat, told Charged. “It doesn’t really offer a huge improvement in energy over a regular lithium-ion battery, but it would presumably offer some safety benefits. It would mainly be a stepping stone to achieving the Holy Grail, where you replace the graphite anode with lithium metal, and you get the huge improvement that everybody wants.”
The challenges of lithium metal anodes
Lithium metal anodes have a high theoretical specific capacity of 3,860 mAh/g (more than ten times graphite’s 372 mAh/g), and a low density of 0.59 g/cm3 (compared to graphite’s 2.26 g/cm3).
“However, using lithium metal with an all-solid electrolyte is really, really hard,” explained Jacobs. “To my knowledge, no one is really close to a commercial application using a lithium anode and a solid electrolyte battery that meets the demanding requirements of an EV.”
“When you have a lithium metal anode, and you strip lithium off, and then you re-plate it over and over again, instead of ending up with a nice piece of lithium metal, you end up with something that looks like a pile of moss and needles and porosity,” Strand explained.
The unwanted growths are known as dendrites. These pointy, branch-like structures form on top of the lithium metal through repeated charge and discharge cycles.
“That causes a lot of problems,” Strand continued. “You’ve got a lot of surface area for a highly reactive material. You’ve got needle-like dendrites that poke through separators and short the cells and, in general, your cells just don’t last very long.”
The challenges of lithium anodes, combined with the challenges of solid electrolytes, make for a very challenging road to the Holy Grail. However, the problems may be easier to tackle one at a time, in Jacobs’s estimation. “That’s why there are two basic paths researchers are taking to solve these issues incrementally.”
The first way is the solid electrolyte and graphite anode approach described above.
The second is to use a lithium metal anode, but find a stable non-solid electrolyte—either a conventional liquid or a semi-solid gel. However, neither option is ideal. Liquid electrolytes see dendrite growth and result in low cycle life. Gel electrolytes are a step better, but just a step, according to Jacobs.
“Gel electrolytes are a solid electrolyte where you actually mix in some amount of volatile, more conventional liquid electrolyte—just a small quantity,” he said. “However, the instant you start to put that in, you’ve eliminated one of the benefits of an all-solid battery, which is the safety component where you eliminate these volatile things. This solution may find a niche market, but isn’t what the mainstream automakers are looking for.”
Wildcat’s research
In 2019, Wildcat was awarded a DOE grant to pursue solid-state technology. Valued at just over $1.22 million, the grant will help fund Wildcat’s promising internal research project—which, due to several years of record numbers of projects with its partners, has taken a back seat to those commercial contracts.
“With the new funding, we have the luxury to be able to apply our high-throughput tools to a more sustained, larger internal effort,” Strand said.
“In addition, we are adding a significant amount of our own capital on top of the DOE grant,” Jacobs added. “Combined, this is now a major emphasis here at Wildcat. It’s a giant program for us.”
Wildcat’s internal research consists of three main thrusts, according to Strand. Two of those thrusts are the stepping stones to the Holy Grail: stabilizing lithium anodes and developing solid electrolyte materials. The DOE grant, intended to further solid-state battery technology, will go towards these efforts.
“We’re trying to address both the energy and safety issues of lithium metal anodes,” Strand said. “Again, the challenge is being able to strip and plate lithium in a way that you don’t end up with a pile of twigs and moss and porous stuff. Towards that end, we’re trying to put protective layers onto the lithium that can hold that material in place and still let lithium strip and plate through the layer.”
As for solid electrolytes, Wildcat is taking a hybrid approach that combines polymers with ceramics.
“It’s hard to find a single material that does well on all of the properties of solid electrolytes. That’s why we’re choosing to go with a composite material that picks and chooses the best properties of polymers with the best properties of ceramics,” Strand said.
Wildcat’s third research thrust is to develop high-energy cobalt-free cathode materials known as disordered rocksalt cathodes.

“We’ve made a lot of really good progress on that cathode material, in terms of improving its overall conductivity or power performance,” Strand explained. “We’re now starting on improvements to cycle life by understanding the fundamental failure mechanisms of that material.”
Eventually, Wildcat would like to see all three thrusts come together in a solid-state battery with a lithium anode and a disordered rocksalt cathode. “But we also believe that each on its own is very valuable to the industry,” Strand said.
New facility, investors, hires and business models
Wildcat’s research is powered by what the company calls its high-throughput platform, or HTP.
“Our high-throughput platform is a combination of lots of different equipment that can take a researcher through the very beginning phase of synthesis of materials all the way through full battery assembly and cycle testing,” explained Wildcat CEO Mark Gresser. “It’s a whole suite of customized equipment that the Wildcat team has designed and built themselves. That’s what allows our scientists to do research in such a unique way.”
In 2018, Wildcat upgraded its HTP alongside a move to a bigger facility in San Diego. The new 23,000-square-foot facility has 65 percent more space to accommodate several improvements to the HTP.
“We’ve put in a new dry room and a prototype pouch cell line,” Gresser continued. “So we have the ability now to make single and multi-layer pouch cells, and this is a great way to validate discoveries coming from our high-throughput platform.”


The improved HTP also features greater automation. “We have a lot of automation, but we improved it all significantly and, most importantly, connected everything through a centralized database,” added Strand. “Now, the scientists can control and monitor equipment from their laptop. They can do all their experimental design and run equipment and execute experiments in a way that they just couldn’t do before.”
An expanded facility isn’t the only new thing at Wildcat. The company recently brought on new investors, including Flint Hills Resources, a Koch Industries subsidiary based in Wichita, Kansas; and InoBat, a Slovakian startup cell-maker.
Wildcat’s business model is to use its HTP to conduct research for its customers. An increasing number of customers, according to Jacobs, are engaging in long-term agreements that span two or three years. These types of new arrangements are ideal for the nature of Wildcat’s research, because the company’s material discovery process can lead to unexpected new opportunities. Long-term partnerships allow Wildcat’s research team to proceed naturally without the need to formalize a new plan at every fork in the road.

Wildcat is considering a new business model: licensing its high-throughput platform.
“Historically, any questions from customers regarding whether they might be able to buy a Wildcat platform of their own, the answer was either no, or you’d have to buy the company,” Jacobs said. “We’re now open to the idea that we might sell a limited number of platforms into the market; this would basically be a license and sale of a complete high-throughput platform.”
To help manage all these new opportunities, Wildcat recently added two industry veterans to its team. The first is Dr. Odysseas Paschos, formerly of BMW’s battery research team, who is now heading up Wildcat’s European business development efforts. The second is Dr. Sun Ho Kang, whose prestigious career took him to Argonne National Labs, Samsung, and Apple before he came to Wildcat.
And Wildcat is still looking to hire. The company is seeking additional help to improve its HTP and meet its internal research goals. “Wildcat is hiring,” Jacobs emphasized. “We’re always looking for more talented scientists and engineers who want to work on cutting-edge battery discoveries.”
This article appeared in Charged Issue 48 – March/April 2020 – Subscribe now.































































![New York City may replace Central Park’s horse-drawn cabs with electric carriages [Updated]](https://chargedevs.com/wp-content/uploads/2025/09/AdobeStock_273233016.jpg)