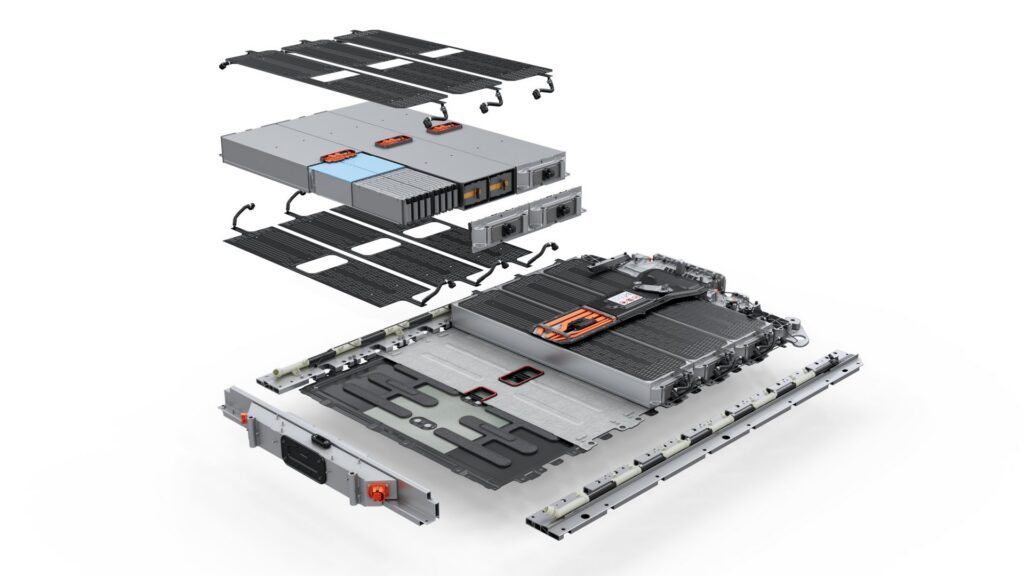
Silicon has a theoretical charge capacity ten times higher than typical graphite. That is why a mind-blowing number of researchers are working towards replacing more and more of the graphite used in today’s lithium-ion battery anodes with silicon.
In the last issue of Charged, we discussed Tesla’s announcement that it had begun to use small amounts of silicon in its cells, and the major technical challenges that must be overcome to increase the amount of silicon in the anode.
The biggest problem for researchers is that, as the amount of silicon is increased, the cycle life of the cell is drastically reduced. This is largely caused by the expansion and contraction of silicon, which undergoes a volume change of up to 300% during charging and discharging. The repeated expansion of silicon essentially cracks the materials around it, which causes all sorts of problems.
In September, we encountered a company that claims to have made significant progress on the road to storing more energy with silicon. Paraclete Energy says its silicon expertise allows it to create a drop-in replacement material that will at least double the capacity of current anode chemistry, with little or no effect on cycle life. To top it off, Paraclete’s silicon products are essentially the same price as graphite.
Charged talked to Paraclete Energy CEO Jeff Norris to learn more about how the company’s silicon technology will live up to the hype.

Charged: What’s your secret? How exactly has Paraclete Energy succeeded where others are struggling?
Norris: We believe that one of the reasons others are struggling with silicon is because most of those companies aren’t silicon experts; they’re battery experts. So there’s been a lot of hype but not a lot of deliverables. Our strength is that we come out of the silicon industry. We know how to make the silicon functional from a cycling perspective. What we’re really selling is our expertise – we partner with customers and help them develop a better anode using silicon.
One of the issues is that, when you have a bunch of smart guys in the room, they tend to want to use the latest and greatest methods to do things. There are a lot of companies using new groundbreaking methods that are really cool, but most of these techniques were recently developed in government labs or university projects, and they’re very exotic. The problem is that the latest and greatest ways to make silicon don’t necessarily create the best or most cost-effective material to improve today’s batteries.

Paraclete Energy started as a philanthropic endeavor. We wanted to manufacture solar panels in third-world countries to help their economies and give them a new energy source. We ended up finding a way to make functional active silicon very inexpensively. We were looking at putting solar manufacturing plants in the remote areas of India, for instance. So the process couldn’t be very energy-intensive, and it had to be done without a clean room and without using inert atmosphere. Those goals led us to a way to make active functional silicon nanoparticles that we can treat for a variety of applications. They can be used in life sciences, biomed, electronics, solar panels – but we decided to focus on batteries because of timing and the potential impact on the industry.
It’s actually very easy to make a silicon-anode battery, but it’s difficult to get it to cycle without capacity loss. The trick is to make the silicon particles very small and cost-effectively, while passivating the surface of the silicon with a compatible and conductive surface modifier that protects the silicon during the charging cycle. We’ve been able to do that using kinetic manufacturing techniques and surface-modifying additives.
Charged: So silicon’s expansion and contraction issues can be solved by making the particles very small?
Norris: That’s right. There are a few published papers by battery researchers – like Professor Yi Cui at Stanford, for example – finding that silicon particles less than 140 nm do not suffer from expansion stress fracturing. This has an impact on cycle life. Also, the surface modifiers we use with the silicon create a covalent bond and communicate with the surrounding graphite. Another problem with silicon’s expansion is that the particles can suffer pulverization from compressive stress when particles are too closely spaced. Our process disperses silicon particles and allows them to stay separated when blended with graphite.
Ultimately, we can add considerable amounts of silicon to existing graphite anode formulations – anywhere from 8 to 25%, depending on the customer. That is enough to double to quadruple the anode’s capacity and experience no noticeable reduction in cycle life – so long as the particles we use are sub-140 nm, bear our custom-tailored surface modifier agent, and are sufficiently separated from each other in the graphic matrix to allow for lithiation expansion.
Charged: Can you tell us more about the surface modifier? How does it work?
Norris: We use a kinetic process to create the silicon particles. If we were to just use pure silicon, without surface modifiers, they would want to bond back together. The surface modifier also prevents the formation of surface oxidation upon exposure to residual oxygen. In many other common processes for creating silicon particles for batteries, a silicon-oxide layer is formed, which is electrically insulating and inhibits reversible lithiation of the silicon particle.
Once an oxide is formed on silicon nanostructure surfaces, it is very difficult to replace the oxide with any other coating. We have seen companies using exotic manufacturing methods of nano-silicon, like plasma deposition, and then some of the lithium is being consumed when breaking through the oxide layer during initial cycling. Basically, the lithium comes from the positive electrode, and has to break the oxide down before it can form an alloy with the silicon. Some of the lithium ions are sacrificed by combining with oxygen, which makes that lithium no longer functional in the battery.
In contrast, our surface modifier is electrically conductive, and it allows lithium ions to penetrate the surface modifier layer and begin to lithiate the silicon suspended in the surrounding graphite matrix. The lithiation process proceeds smoothly without wasting any lithium.
Charged: So why don’t these other processes for manufacturing silicon nanoparticles also use surface modifiers?
Norris: Again, this is a problem with the exotic methods that everyone uses to make their functional silicon. The plasma deposition method, for example, forms silicon into nanoparticles at over a thousand degrees Celsius. At those temperatures, our surface additives are unstable and would instead form a silicon-carbide surface. We use room-temperature methods to make the silicon, so we can add a custom surface modifier without any problem of thermal degradation.
![]()
![]()
If you look at the shape of our particles versus those made with other methods, you can see a very clear difference. Plasma deposition and other thermodynamic methods tend to make spherical particles. Ours look like pancakes. It’s all about the surface, and we prepare the silicon surface specifically for our customers’ chemistry. For example, if our silicon nanoparticles are going to be used with an NMC cathode or lithium-iron phosphate cathode, they get a different surface modifier.
![]()
Charged: How do your partnerships with battery companies work? What do you supply them?
Norris: We think of ourselves as integrators and project-oriented silicon experts. We try to take the customer’s status quo battery and make it better. So, we work side by side with partners to make their proprietary formulations better.
A battery manufacturer will give us their preferred formulation of its battery, or something very close to it, and then we’ll prove that we can at least double the capacity of that anode. We optimize our coatings for the silicon nanoparticles to work best with the particular battery formulation, and the battery company gets three things in return.
They get batteries back that we’ve made using the same manufacturing process they use, but now they also contain silicon nanoparticles. We also give them all of the new performance data showing that if they take some fraction of their material away and replace it with our material, this will double to quadruple the capacity of their anode.
![]()
Another deliverable that we provide is a standard operating procedure (SOP) that tells them how to add the silicon nanoparticles to their manufacturing process and keep everything else the same. The beauty of our approach is that it can be implemented with almost no change to their manufacturing process. Just mix our anode additives into the slurry and make the electrode. As simple as adding creamer to coffee, or, more specifically, similar to the way they add binder to their slurries. So, we give them an SOP that describes exactly how to mix in the silicon with their chemistry.
That process will increase their anode’s energy density by some amount. It could be two times, three times or more, depending on the chemistry.
The third thing we deliver is another set of SOPs that outlines how the company could change their current manufacturing process in order to get even better performance. We may suggest things like resting the electrode sheet, increasing the temperature, using different solvents in the slurry process, or changing the electrolyte.
After we deliver those three things, customers usually start by trying to replicate the results we’ve proven in a pilot at their own facilities. So we provide materials and coach them on the process. The final stage is to commercialize the new formulation and manufacture it at scale.
We’re still a young company, founded in 2010. All of our projects are in the R&D or pilot phases, and the reception so far has been incredible. I would say the best-case scenario for one of our projects to be commercialized is next year by a Korean battery company. But that depends on how fast the customer moves, it’s up to them.
We also offer long-term support options that give access to our engineers and scientists. And we have new materials developing in the lab that are showing even higher performance than what we’re using now. We’ll eventually bring that to the market and if you’re on our licensing agreement, you get it too.
Charged: Do you see a path to eventually using an anode made of 100% silicon, or close to 100%?
Norris: Yes, but it will be gradual. A lot of people have experimented with completely replacing the anode with silicon, and they run into all sorts of trouble.
So we’re off to a great start by doubling to quadrupling the capacity of the anode with just a fraction of silicon. When you also consider that it’s essentially the same price as the graphite they’re already using, and there is no loss of cycle life, and you don’t have to add any new processes to your manufacturing facility, it’s a no-brainer to start with a smaller fraction and gradually increase it.
In the future the next step is maybe 30, 45, or 50% silicon, with the eventual goal of adding as much as possible to achieve 10 times the capacity.
This article originally appeared in Charged Issue 21 – September/October 2015. Subscribe now.