Imagine an EV that could add 300 miles of range with a 5-minute charge – roughly the same amount of time it takes to refuel an ICE vehicle. Many companies are working on technologies to enable faster charging, which would be a huge win for EV adoption.
ZapGo, based in Oxford, UK and Charlotte, North Carolina, believes its energy storage technology is critical to unlocking that future. Since 2013, the company has been developing carbon-ion (C-ion) technology that it says looks past the limits of traditional Li-ion batteries and supercapacitors.
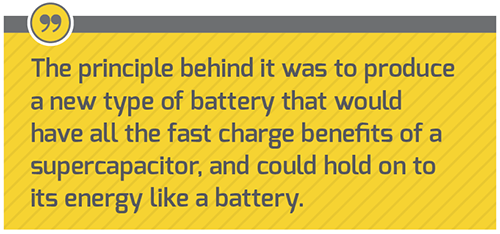
“I have a background in energy storage, and about five years ago I came across some intellectual property developed at the University of Oxford,” Stephen Voller, founder and CEO founder of ZapGo, told Charged. “The principle behind it was to produce a new type of battery that would have all the fast charge benefits of a supercapacitor, and could hold on to its energy like a battery. That’s what we’re now developing.”
Carbon-ion: combining the power density of supercapacitors and the energy density of batteries
As ZapGo sees it, currently available technology leaves a lot of room for improvement. Li-ion batteries have evolved into very capable energy storage devices – which accounts for their near-universal adoption. The Li-ion battery market is on track to be worth $140 billion by 2026. However, ZapGo thinks we’re approaching the limits of the technology based on the constraints of Li-ion, including relatively slow charging rates and finite lifespans.
On the other hand, electrical double-layer capacitors (EDLCs) – aka supercapacitors – have a much longer life and can charge and discharge much more rapidly, but can only store a small amount of energy per charge compared to Li-ion cells.
ZapGo says that its new C-ion technology falls between these two commercially available products and transcends their limitations.
Structurally, C-ion cells are very similar to EDLCs. “With batteries you have electrochemistry that’s going on,” explained Voller. “When you charge and discharge there are chemical reactions inside that literally wear out over time. With a supercapacitor, you have an ion-electric reaction – it’s more like static electricity. So there’s no chemistry going on to wear out.”
“The key to the differentiation of our technology is that we use different materials than the conventional EDLCs and Li-ions. We use nanocarbons as opposed to activated carbons. And we use ionic electrolytes as opposed to organic electrolytes. We’re really a materials science company.”
ZapGo says that recent advances in producing and fabricating a range of nanostructured carbons and ionic liquid-based electrolytes have made C-ion cells feasible.
Different types of nanocarbons are now commercially available in the form of powders, microspheres, fibers, foils and monoliths. The physical and chemical properties of synthetic and nanostructured carbon materials such as graphene and single-walled and multi-walled carbon nanotubes are ideal for energy storage because they have large surface areas and unique nano-structures.
More specifically, some have suggested that the theoretical upper limit for the specific capacitance of a graphene-based electrode is as high as 550 farads per gram (F/g). In the lab, by matching the pore size of nanocarbon to the ion size and optimizing with the best ionic liquid electrolyte, scientists have been able to create supercapacitors that are able to store 160 F/g.
In the real world, this has led to the development of C-ion cells that are currently able to store five to six times as much energy per kilogram as commercially available supercapacitors.
These advances are due not only to the use of nanocarbons, but also to the incorporation of ionic liquid electrolytes, which are stable at higher operating voltages than organic electrolytes commonly used today. Increasing the operating voltage of a cell directly increases its energy density, and ionic liquid electrolytes have been shown to be stable at voltages as high as 6 V, about double the limit of typical organic electrolytes. However, they also come with design challenges.
Ionic liquids with a large electrochemical window tend to be several times more viscous than organics. This leads to a lower ionic conductivity, higher internal resistance and compromised power characteristics. During heavy power demand, the cell or a stack of cells will struggle to deliver huge power in a quick spurt.
However, ideally-designed electrodes help to overcome these challenges. Figure 1 shows a traditional supercapacitor design and the transport of ions in 2D geometry. An ion at one of the electrodes located close to the current collector has to migrate through the entire electrode and separator material to reach the other electrode while being charged or discharged. Due to the thickness of the carbon electrode and the separator, it can be a long and winding path for the ion to move from one electrode to the other.

However, by using synthetic carbons and nanocarbons, it is possible to fabricate electrodes with controlled porosity lattices. Figure 2 illustrates the precisely designed pores of a C-ion cell, which allows for a more direct path for the ion transfer.
ZapGo says that in recent years, the combination of advances in nanostructured carbons as electrodes and non-flammable ionic liquids as electrolytes has significantly enhanced the performance of C-ion cells. These new carbon and electrolyte materials not only operate at higher voltages, enabling higher energy densities, they’re also much safer and easier to recycle.
ZapGo is focusing its current research efforts on developing gel and all-solid state C-ion cells. Specifically, the company is creating polymer-inorganic composite electrolytes in the form of membranes. These materials are tailored to contain interconnected nano-sized channels formed by the polymer network for easy ion migration. The polymer network weakly binds the ions to enable fast ion transport. The weak binding and fast ion transport is achieved by creating a network of vacant binding sites in the polymer.
Producing today, innovating for tomorrow
ZapGo is currently on the third generation of its carbon-ion technology, which will go into production this year, to be used in consumer appliances such as hand tools and electric lawn mowers. Those cells will offer a specific energy of 13.4 Wh/kg, 2.4 Wh of stored energy, fast charging rates and a lifetime of more than 100,000 cycles.

Simultaneously, the company is also iterating a next generation of cells designed to store more energy, with an eye to the EV and grid storage markets. ZapGo says its fourth-generation cells will have a specific energy in the 32-56 Wh/kg range. As it moves into generation five and six C-ion technology with 5 V and 6 V cells, the company says it’s confident that the design of a battery pack using C-ion technology will be able to meet or exceed the typical pack-level density found in vehicles of 100 Wh/kg and above.
As with many other new battery technologies we’ve covered, one of the biggest challenges is translating great results in the lab into full-scale production. ZapGo says a major focus was to create a carbon-ion cell that can be manufactured on the same assembly lines, and with much of the same materials, as Li-ion cells. The cells that will go into production this year will be produced in a factory that already makes Li-ion cells.
“The first thing was to make sure that we could manufacture this technology at scale,” Voller said. “There are literally billions of batteries made every year. And you can’t, as a new technology, just get into the field if you’ve got to reinvent the wheel. So the first thing we wanted to do was ensure that we could make [the cells] ourselves using the existing Li-ion manufacturing. The first generations of the technology are really about proving that as a concept.”
How much?
Perhaps the most important question for automotive energy storage is: what will it cost? As for the price of C-ion batteries, Voller expects the cost to be comparable to – if not better than – Li-ion because of the basic building blocks of the cells.
“A figure that’s often quoted for Li-ion is $100/kWh,” he explains. “And that’s a figure that we expect to meet or exceed for our customers as we move into volume. We can be confident about that because we take away two of the most expensive elements currently in Li-ion cells, which are the lithium and the cobalt. We don’t use any of that, but we do use the existing manufacturing techniques and the rest of the supply chain.”
Road map
ZapGo has ambitious plans for the future of C-ion. “We think there will be a two-step process,” Voller predicts. “The first step is that there would be a hybrid of the existing lithium batteries and our cells. Williams Advanced Engineering, part of the Williams Formula One team, are developing a control system to allow a vehicle to use C-ion and Li-ion side by side on the same vehicle. The second stage would be to replace the lithium completely, because it would be much safer and much cheaper.”

ZapGo says it’s hard at work on R&D to reach the second stage of the process. Specifically, the company is trying to increase the operational voltage of carbon-ion, which directly relates to its energy density.
“Our current third-generation cells operate at 3.4 V,” Voller explains. “To replace lithium-ion on a vehicle and get to the same sort of energy density, we need to get to 5 or 6 volts in the cells. And we have a roadmap to do that over the next few years. So we expect that we will produce cells that can meet or exceed the energy density of lithium-ion, and provide a safer and faster charging experience for the driver.”
Improving safety improves designs
Without organic electrolytes and lithium, ZapGo says that C-ion cells are much more stable and safer, designed to comply with both current and future shipping regulations (today, transporting Li-ion cells is a regulatory nightmare). It also means that there is significant potential to greatly simplify the design of EV battery packs. Because of the stringent safety concerns, engineering EV battery packs is costly.
ZapGo is also quite enthusiastic about an interesting design possibility that the safety of C-ion could enable: embedding electrodes in structural components like the vehicle chassis.
“The BMW i3 has a carbon fiber chassis, so there’s no steel in the vehicle at all,” Voller says. “Imagine that chassis now becomes the energy storage. So instead of having individual cells, we make entire structures or panels that become part of the vehicle. And the benefit of doing that is to take orders of magnitude of cost out of the vehicle. We could do that because our technology is fundamentally carbon, and the technology that BMW and others use is carbon fiber or lightweight composite structures that can be incorporated into the electrodes of our cells.”
“It wouldn’t be a door panel, because obviously you need to provide electrical protection. But you could fabricate a chassis segment, for example, that might be a 10 kWh, 48 V panel that would have just two connections: the positive and the negative to the vehicle bus.”
Exotic application possibilities aside, with C-ion cells, pack engineering could be greatly simplified, further increasing its competitiveness on a cost and energy density per pack basis.
Stationary systems
The two biggest advantages of C-ions in EVs would be fast charging and long life. Although many of the next generation of vehicles are being designed with Li-ion cells to support charging rates as high as 350 kW, this is still a far cry from the power that would enable 300 miles in 5 minutes.
“Put simply, if you plugged an EV with Li-ion into a 1 MW charger, it would catch fire pretty spectacularly and quite quickly,” Voller said. “So you need a different technology on the vehicle to be able to accept that amount of energy that quickly. And that’s where we’re working with the automotive companies towards the next generation of vehicles that are capable of providing the driver with the same experience that they get today with gasoline.”
There are also enormous opportunities for C-ion cells to be used in the fueling stations of the future. Next time you’re at a busy highway gas station, image that all those vehicles were recharging 300 miles of range in 5 minutes – one after another, all day and night. The amount of power and energy delivered would be eye-popping. In the future, those stations will need large-scale energy storage that can recharge during off-peak times and meet everyone’s needs during rush hour. This seems like a great fit for technologies with high peak power and extremely long cycle life.
This article originally appeared in Charged Issue 39 – September/October 2018 – Subscribe now.