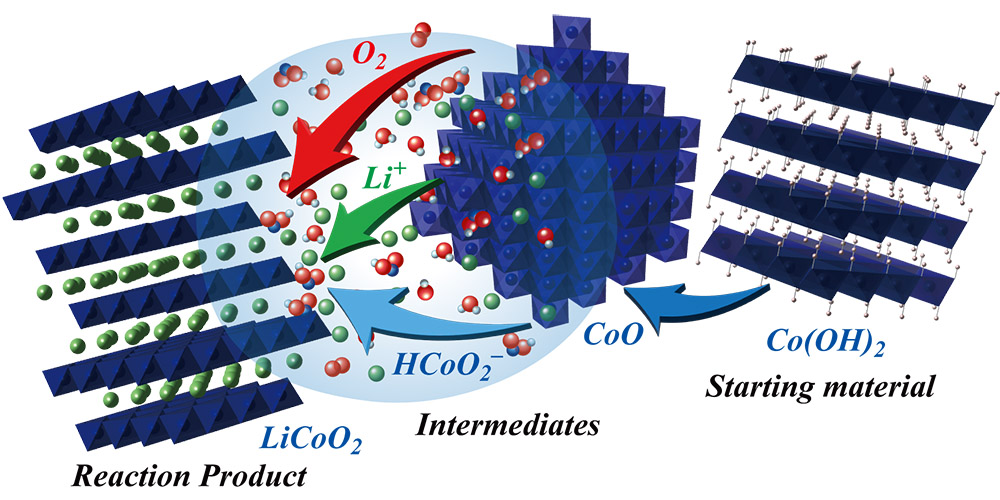
Layered lithium cobalt oxide (LiCoO2), a key component of lithium-ion batteries, has been synthesized at 300° C in 30 minutes by a team of researchers at Hokkaido University and Kobe University.
Lithium cobalt oxide is used for the cathode in lithium-ion batteries and traditionally, the synthesis of this compound takes 10-20 hours to complete at 800° C. The research team, led by Professor Masaki Matsui at Hokkaido University’s Faculty of Science published the findings in the journal Inorganic Chemistry.
Using cobalt hydroxide and lithium hydroxide as starting materials, with sodium or potassium hydroxide as an additive, the team conducted experiments under varying conditions to synthesize layered lithium cobalt oxide crystals using what they call a hydroflux process. The team also measured the electrochemical properties of the material, and found that they were only marginally inferior to that of lithium cobalt oxide synthesized by the traditional high-temperature method.
“By understanding the reaction pathway, we were able to identify the factors that promoted the crystal growth of layered LiCoO2,” Matsui said. “Specifically, the presence of water molecules in the starting materials significantly improved crystallinity of the end product.”
Source: Hokkaido University