The current generation of lithium-ion battery materials is quickly approaching its theoretical limit. In commercial layered oxide cathodes, like the popular nickel-manganese-cobalt (NMC), there is only one lithium ion for every metal. So, the most energy you could possibly store with those materials is one full lithium in and out, or about 300 mAh per gram of cathode.
Today’s commercially available cells are cycling about 0.6 to 0.7 lithium ions per metal, and the market is steadily increasing that ratio by using more nickel- and manganese-rich compositions. Industry insiders report seeing several materials that are closer to 0.8 to 0.9 lithium ions per metal, and the lithium-rich NMC materials – pioneered by Argonne National Laboratory – are basically cycling the full amount of lithium. In other words, the headroom for improvement is quickly dropping.
That reality has spurred a lot of research into next-generation cathode materials like lithium-air, lithium-sulfur and metal fluorides.
Last year, Charged reported that Wildcat Discovery Technologies is developing new techniques to overcome the shortcomings of a new copper fluoride formula that it calls CM4. Metal fluorides can have very high energy densities, but are known to have problems with low power density and poor cyclability. The Wildcat team thinks they see a path to improving these pitfalls in copper fluoride.
Now, with a major cell manufacturer as a development partner, the researchers are sharing more details.
High Speed Science
Wildcat Discovery Technologies uses proprietary methods to rapidly synthesize, test and evaluate energy storage materials. The venture-backed start-up, based in San Diego, uses parallel development capabilities similar to the way discoveries are made in life sciences, such as pharmaceuticals. In the area of batteries, however, Wildcat is one of the only organizations that can synthesize useful storage materials in bulk form and then screen those materials very quickly to find out how useful they are.
The company uses its unique skills in collaborative research projects with many different chemical companies, cell manufacturers and even electronics and vehicle OEMs. It also funds its own internal projects when a new material seems particularly promising, like the copper fluoride CM4 cathode work.
To address the power problem, the company applied a newly developed molecular coating technique that is similar to carbon coating. Carbon coating can significantly increase the conductivity of electrodes, but cannot be used with many metal oxides and fluorides because the materials are reduced at the high temperatures required for coating. Wildcat found that when using its new coating process with copper fluoride electrodes – originally developed while working on carbon fluoride primary batteries – the result is a high-capacity, high-power electrode.
“We’ve developed a non-graphitic conductive coating, different from the traditional carbon coatings like that used with lithium iron phosphate,” said Steven Kaye, Wildcat’s Chief Science Officer. “It enables these metal fluoride materials to have similar rate capability and power to commercial layered oxide cathodes. We’re getting great results. Instead of having to discharge cells in 50 hours, we can discharge them in 30 minutes or less.”
To improve cycle life, Wildcat uses another special protective coating that offers “a very large improvement” over the state-of-the-art copper fluoride technology, although there is still work to be done to get it to a more practical level. “We think we’ve got a technology with a path to the cycle life you’d need for commercial applications,” said Kaye. The company has been tweaking the electrolyte and the composition, which is also helpful, but the big technology leaps are the coatings.
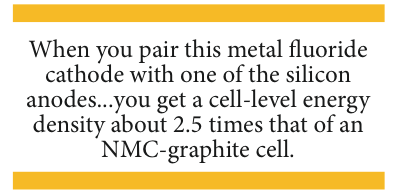
The reason that Wildcat and its new development partner are excited by these advancements is that when you pair this metal fluoride cathode with one of the silicon anodes, currently showing real promise in the lab, you get a cell-level energy density about 2.5 times that of an NMC-graphite cell.
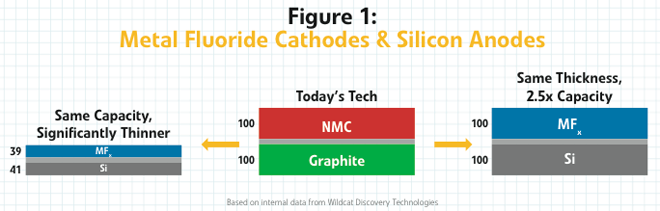
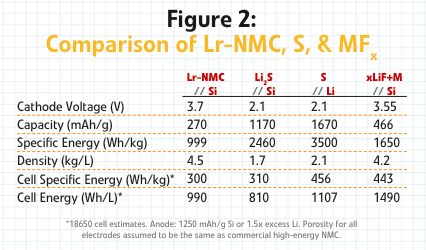
Today’s cathodes in 18650 consumer electronics cells get approximately 650 to 700 Wh/L. With this copper fluoride material, you can get somewhere between 1,500 and 1,600 Wh/L with reasonable expectations for a silicon anode,” explained Kaye. “That’s on par with the lithium-sulfur systems that people are very excited about, except you don’t have the problems with power or sulfur dissolution.”
Silicon offers a much higher capacity than graphite, which means the anodes can be made thinner. But many silicon anodes also have cycle life problems, for two main reasons: the expansion and contraction of the silicon; and the inability to form a stable solid-electrolyte interphase (SEI) at the surface of the silicon. The electrolyte needs to coat the surface of the anode to protect it – a protective coating is very stable with graphite but not with silicon.
However, Charged has reported on silicon anode prototypes with a cycle life that are very close to what is needed for consumer electronics, from several cell makers. At this point, it’s a basic trade-off between the amount of silicon in the anode and how many cycles they can achieve. There are cells with a few percent silicon that could probably be used today, but the capacity boost is not great. On the other hand, there are cells with 6-10 percent silicon and a considerable capacity boost that are very close to being commercial-ready.
Next step: more partners
Wildcat has been in the battery materials R&D business for about four years, and CEO Mark Gresser says that research spending continues to increase. That’s not surprising when you look at the markets using Li-ion technology today, like electric vehicles and consumer electronics – both growing very fast.
The interesting part about battery research is the diversity of companies involved in it. “OEMs are doing research, cell makers are doing research, and materials and chemical companies are doing research,” said Gresser. “They are spending money on R&D at all parts of the supply chain, which is unique to this industry.”
Wildcat is largely agnostic to any particular battery chemistry, application or stage of development – it will work with anyone. So, even as some companies go out of business and others are acquired or shift focus, its business continues to grow.
Because of the nature of Wildcat’s research methods, a development project can move much faster with more funding. So, the company is actively seeking more partners for its copper fluoride project. “We’ve brought on a major cell manufacturer as a partner,” said Jon Jacobs, VP of Business Development. “Their expertise with-large format battery systems manufacturing will really speed the development of this material to market. Next, we’d like to add a major automotive OEM to the consortium to provide additional funding and expertise.”
This article originally appeared in Charged Issue 13 – APR 2014