Mike Zimmerman, CEO and founder of Ionic Materials, has this to say about his company: “Ionic will play a major role in the solution to the world’s energy problems.”
That’s a pretty bold statement. But then again, Mike Zimmerman is a pretty bold man. An experienced polymer scientist, Zimmerman has worked on a number of ground-breaking technologies, from the first fiber-to-the-home system in electronics to the first plastic package for semiconductors. He started and sold a successful materials science company called Quantum Leap Packaging, and has spent the last 25 years teaching materials science at Tufts University.
But all that was only the prelude to Ionic Materials.
“For some reason, five years ago I started thinking about batteries,” Zimmerman says. “I wasn’t a battery person, but I started looking at the materials of a battery. I saw that a lot of the improvements were being made on the anodes and cathodes, and I noticed that the electrolyte was a real limiting factor.”
With that one serendipitous observation, Zimmerman had begun his journey to help solve the world’s energy problems.
The trouble with liquid electrolytes
“Since about 1990, the major rechargeable battery has been a lithium-ion battery,” Zimmerman told Charged. “And it had a liquid electrolyte. And I said to myself, you probably couldn’t put together three worse materials: reactive anode, cathode, and this very flammable liquid electrolyte.”
By now, the hazards of liquid-electrolyte lithium-ion batteries are pretty well known to the public. Even though they’re ubiquitous in all sorts of devices, from personal electronics to EVs, they do occasionally short-circuit and catch fire. In 2016, for example, smartphone manufacturer Samsung was forced to recall 2.5 million Galaxy Note 7 phones after reports of fires resulting from manufacturing defects. A quick YouTube search will also reveal video after video of what happens if lithium-ion batteries are punctured (spoiler alert: smoke, smoldering, flames and the occasional rapid release of gases – i.e. explosion).
One approach to this problem is to swap the flammable liquid electrolyte for something more durable: a solid. And although this is the approach Zimmerman took, he was not the first to try it.
“There’s been two classes of solids, ceramics and glasses,” he explains. “And they all have their challenges. One of the big challenges with both is to make them thin and big. Ceramics are very brittle, so it’s hard to scale them up. And you can imagine even putting glass in an electrolyte is a difficult challenge.”
Zimmerman, with his background in polymer science, saw the potential advantages of developing a solid polymer electrolyte. Before he began his work, there was only one such electrolyte: a polymer called polyethylene oxide. Although this substance can transfer ions, it suffers from two major drawbacks: it’s only conductive at high temperatures, and it’s not compatible with high voltages.

“So I said, being a polymers person, it would be very beneficial if somebody could develop a polymer that could be extruded, and used plastics processing technology that could actually function appropriately at room temperature. So I started working on a polymer electrolyte that could be as functional as a liquid electrolyte but would solve a lot of the problems of a liquid electrolyte, mostly safety and energy density.”
The solid solution
Through his efforts, Zimmerman managed to develop a solid polymer which addressed the limitations of polyethylene oxide – and opened the door to a better type of battery.
“What I did as a polymer scientist was to come up with a material which has a completely different conduction mechanism,” Zimmerman explains. “It doesn’t require movement of the chains. The conductivity at room temperature is equivalent to that of a liquid electrolyte with a separator. And the polymer is processable like in roll-to-roll, so it’s highly manufacturable. That’s what separates us from everybody else.”
In a conventional lithium-ion battery, two electrodes – the anode and cathode – are on either side of a separator, which prevents a short circuit while allowing ion transfer. The liquid electrolyte, which conducts the ions, surrounds each electrode.
Zimmerman’s new material allows for a unique battery architecture. “We’re replacing both the separator and liquid electrolyte with polymer,” he says. “So the ionic polymer does two functions: it’s electrically insulative, so it acts as the separator, and it’s ionically conductive, and acts as the electrolyte.”
This approach creates a completely solid-state lithium-ion battery, which unlocks a number of benefits. Chief among them: safety.

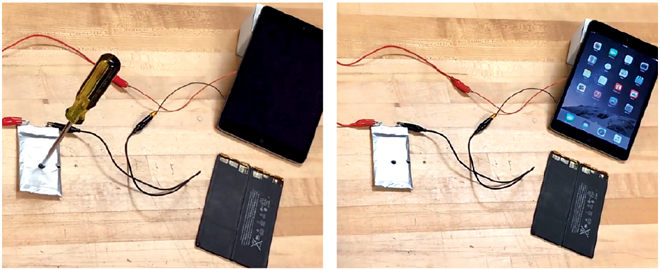
“You can shoot bullets through a battery made with our material and it won’t explode, it won’t burn, and it still works afterwards,” claims Zimmerman. But don’t take his word for it – you can actually go to the Ionic Materials website and watch a video of exactly that. The technology was also featured on a recent Nova documentary, Search for the Super Battery, in which you can see Zimmerman’s battery get cut, stabbed, and burned without giving off so much as a single spark (and while remaining completely functional).
But the advantages don’t end there, according to Zimmerman.

“People are trying to go to higher energy density anodes and cathodes,” he says. “And another major benefit is that we’re enabling electrodes made of lithium metal, which has much higher energy density.” One of the major challenges with lithium metal electrodes comes in the form of dendrites, fingerlike projections of metal that build up from one electrode, harming performance and eventually creating a short circuit. Replacing liquid electrolytes with an affordable and functioning solid creates a physical barrier that will prevent dendrite formation.
A third benefit of Ionic Materials’ approach is that it has the potential to lower the cost of batteries. For one thing, there are some cost savings that can be attained by using plastics manufacturing and eliminating the liquid electrolyte. For another, the new polymer can enable certain alternatives to lithium-ion chemistries, such as rechargeable alkaline, which uses cheaper electrodes than lithium-based batteries do.
All of this has the potential to make a big impact in the world of EVs, as Zimmerman points out.
“What’s going on is the automobile industry is really in a push to turn cars from internal combustion to batteries,” he says. “And in order to do that, they’re working on several things: range, cost, and safety. And our polymer has a great chance to solve the range, the cost, and the safety issues with traditional lithium-ion.”
A ringing EV endorsement
Despite the demonstrable success of Ionic Materials’ new solution, don’t expect to see the technology on the road just yet.
“I think shorter-term it will probably be in some consumer products – wearables or cell phones,” Zimmerman says. “But there’s a big need to shortly have the technology in prototype batteries for electric vehicles. It takes a long time to commercialize electric vehicles and new batteries. We’re working with battery companies to provide initial prototypes in 2018, but there’s a long qualification cycle before it actually gets into production.”
Ionic Materials does not plan to be a battery manufacturer itself, but rather to partner and license its technologies to the full-scale production experts. It’s currently connecting with cell manufacturers and end users that are interesting in using its technology.

The company recently got a boost in this endeavor from a formidable source: the Renault-Nissan-Mitsubishi Alliance. Earlier this year, the automotive powerhouse and worldwide leader in EV sales launched a new corporate venture capital fund, Alliance Ventures, which is slated to invest up to $1 billion over five years. Its very first move? To invest in none other than Massachusetts-based Ionic Materials.
It seems that Zimmerman has got some big players in his corner, even if he and his company still have a lot of work to do. He explains that development for this type of battery material is never really done. The next steps include a lot of reliability testing and scaling-up efforts. But as Zimmerman says, he isn’t one to back down from a challenge.
“The technical hurdles Ionic proposes to overcome are significant, but such challenges we choose to accept, as the world needs our solution.”
This article originally appeared in Charged Issue 35 – January/February 2018 – Subscribe now.