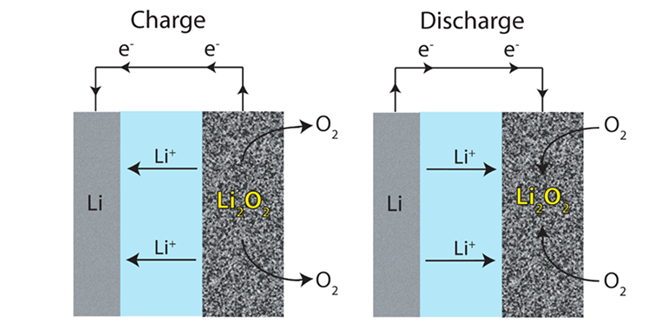
Lithium-air batteries are widely expected to be the next big thing – more than 300 research papers have been published on the topic in the past 3 years. Li-air has the potential to deliver far more energy density than current lithium-ion technology.
A new review in the ACS journal Chemical Reviews sums up the current state of the quest, and focuses on the most critical issues that must be addressed for Li-air batteries to be commercialized.
“Development of a practical Li-air battery will involve overcoming many formidable challenges, including the need for a fundamental understanding of Li-O2 electrochemistry, development of new and improved cell materials, and innovation in the critical aspects of cell design,” wrote Jun Lu, leader of a team of researchers from Argonne National Laboratory, Beijing Institute of Technology and Hanyang University.
A Li-O2 cell has a theoretical energy density of around 11,680 Wh/kg—not much lower than that of gasoline (13,000 Wh/kg). Practically, however, the energy density of the batteries is far less.
“The usable energy density of gasoline for automotive applications is approximately 1,700 Wh/kg, assuming an average tank-to-wheel efficiency (12.6%) of the US fleet,” according to the paper. “Fortunately, such energy density accounts only for 14.5% of the theoretical energy content of a fully charged Li-O2 battery, so it is not inconceivable that such a high energy density may be achievable at the cell level.”
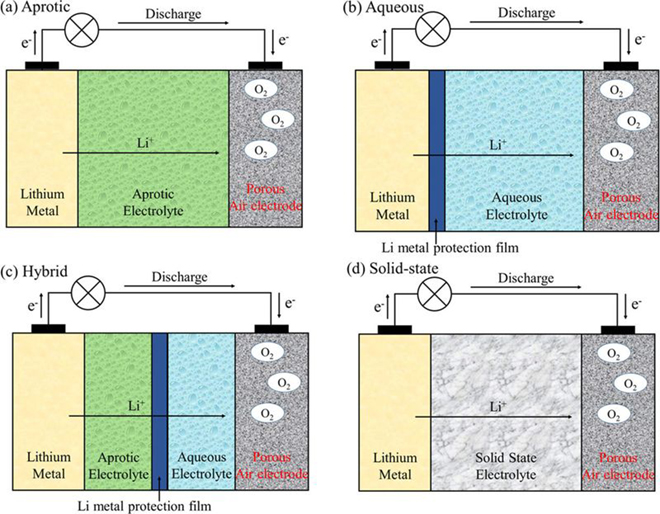
There are four types of Li-O2 batteries under development, characterized by the type of electrolyte: aprotic; aqueous; solid-state; and hybrid aqueous/aprotic. In the review, the team focused on aprotic and aqueous Li-O2 systems.
Image Credit: ACS, Lu et al
A typical aprotic Li-O2 cell consists of a lithium electrode, an electrolyte consisting of dissolved lithium salt in an aprotic solvent, and a porous O2-breathing electrode that contains carbon particles and, in some cases, an added electrocatalyst. The greatest challenge is the search for stable electrolytes. While carbonate-based electrolytes have been used in much research work, these electrolytes decompose in the presence of the superoxide radicals. Ether-based electrolytes seem to be relatively stable in the presence of the reduced oxygen species; however, their stability during charge, especially at high voltage, remains unclear.
“Without question, searching for a fully stable electrolyte in the oxygen-rich electrochemical environment is the research priority at present,” say Lu et al. “Design of a robust strategy for effectively screening the stability of various electrolytes would be greatly beneficial to the development of a Li-O2 battery for practical applications.”
For aqueous systems, the team found that a better understanding of Li-O2 electrocatalysis is required, since the Li-O2 electrochemistry is unique and different from that of conventional electrocatalysis. “The successful development of any aqueous Li-air batteries relies on the prevention of direct contact of the lithium metal electrode with water. The most innovative approach to address this issue is the introduction of Li-ion conducting glass ceramics. However, these ceramics are generally fragile and highly resistive at low temperature. Moreover, they may not be very stable in strong acidic or basic media. Future research and development of large and more flexible LiC-GC membranes will be greatly beneficial to the aqueous Li-O2 system.”
Source: Green Car Congress
Top image by Wikimedia Commons user Na9234