Aging is an inescapable fact of life, not only for people, but for batteries as well. Researchers at the Helmholtz Center Berlin (HZB) recently identified a major reason why Li-ion batteries degrade with time. They have directly observed atomic rearrangements occurring in cathode materials during charge and discharge processes, which lead to the breakdown of the crystal structure of a material.
Dr Jatinkumar Rana and his colleagues are studying electrochemical processes in high-capacity Li-rich cathode materials. The cathode material is (x)Li2MnO3*(1-x)LiMO2, and “M” is a transition metal like Manganese, Chromium or Iron. These materials deliver twice the capacity of commercially available cathode materials, and can be charged or discharged in short times or at very high currents. “Besides, they contain lesser amounts of rare, toxic elements such as Nickel and Cobalt, which makes them cost-effective and eco-friendly,” said Dr Rana.
However, Li-rich cathode materials suffer from a reduction in battery voltage upon cycling, known as the “voltage-fade” mechanism, which reduces energy density. Also, there remains a fair amount of ambiguity about the role of the Li2MnO3 component in electrochemical processes of Li-rich materials, since it is believed to be electrochemically inactive. “The answers to these questions lie in thoroughly understanding electrochemical processes and associated structural changes in Li2MnO3” explains Rana.
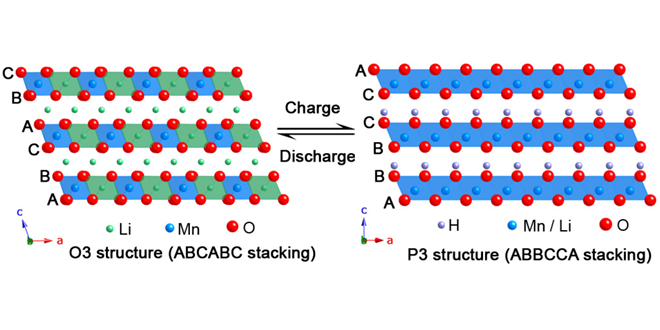
The scientists investigated charged-discharged samples of Li2MnO3 during the first and 33rd cycles by X-ray absorption spectroscopy (XAS). “The element selectivity of XAS provides a unique opportunity to probe electronic, chemical and structural changes occurring at and around individual atom types in a material,” said Rana. “Such a combination of element-specific information is rather difficult to obtain from X-ray diffraction, which provides average changes in long-range structure of a material.”
The team observed oxygen removal from the material during the first charge and shearing of oxygen layers as a result of the Li+-H+ exchange. “The cumulative effect of such repetitive shearing of atomic layers during cycling is that the material gradually loses periodicity in atomic arrangements and, as a result, the electrochemical performance of a battery degrades upon cycling,” said Dr Rana.
The observed structural changes in Li2MnO3 provide vital clues about the mechanism of electrochemical activation in Li-rich cathode materials. “A series of Li-rich cathode materials so far investigated by us show similar structural changes as observed in the case of Li2MnO3. Now that the electrochemical processes in Li-rich cathode materials are becoming clearer to us, we can use this knowledge to improve the cycling performance of these cathode materials,” said Dr Rana.
Source: Helmholtz Zentrum Berlin via Green Car Congress